Understanding the Correlation
Understanding the Correlation While the relationship is not causal, the presence of periodontal inflammation may impact the cognitive degeneration associated with Alzheimer’s disease.
This course was published in the November 2015 issue and expires November 20, 2018. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the signs and symptoms of each stage of Alzheimer’s disease (AD).
- Identify the risk factors for developing AD.
- Explain the potential for periodontal bacteria and systemic inflammation to contribute to the development and progression of AD.
- List the important components of a dental hygiene care plan for patients with AD.
A healthy brain includes more than 100 billion neurons that send information through chemical bursts across 1 trillion synapses to other neurons. AD disrupts the brain’s ability to communicate via healthy neural transmission, leading to the destruction of memory and cognition.3 Classic biological hallmarks of AD are the presence of beta amyloid plaques and neurofibrillary tangles in the brain tissue.4 Beta amyloid plaques are formed when fragments of amyloid precursor protein clump together outside a neuron, preventing information from being passed across a synapse and interfering with the neuron’s function. In healthy brain cells, the tau protein stabilizes and holds the microtubules (used to transport proteins) together. In a patient with AD, the abnormal tau protein separates from the microtubules, causing the tubules to fall apart and form tangles inside the neuron.3,5 These tangles block the transport of nutrients and other molecules into the neuron. Once the neuron is blocked from communicating across synapses by neurofibrillary tau tangles or beta amyloid plaques, the neuron begins to shrink and eventually dies. As more neurons die, the brain loses its ability to communicate, process information, and store memories. Brains affected by AD show considerable shrinkage and debris from cell death and dying neurons. This process can begin as many as 20 years before symptoms occur.3
AD is the most common type of dementia. It is characterized by irreversible memory loss and impaired judgment. Early-onset AD affects individuals between the ages and 30 and 40 and is rare. Late-onset AD occurs in those age 65 and older and is the most common. The majority of patients with AD live for approximately 8 years to 10 years after diagnosis, but some can live 20 years or longer with the disease. AD can be broken into four stages of development: early, middle, advanced, and terminal (Table 1).6
POTENTIAL CAUSES AND RISK FACTORS
Although the biologic transformation that occurs in the brain due to AD is understood, the exact causes and risk factors are still being researched. Risk factors for AD include: age (typically over 65), family history (especially diagnosis of a first-degree relative), mild cognitive impairment, traumatic brain injury, cardiovascular disease, smoking, obesity, diabetes, high cholesterol, hypertension, lack of social and cognitive engagement, low levels of formal education, and genetic variations. The most common genetic variation is the apolipoprotein ε-4 (APOε-4).3 The APO? gene provides the instructions to develop lipoproteins that transport cholesterol in the bloodstream. Maintaining normal levels of cholesterol is essential in preventing cardiovascular diseases.7 All individuals inherit an APOε from each parent. The most common pairing is two APOε-3 genes, which has no effect on developing AD. Researchers believe having two copies of the APOε-2 gene decreases the chance of developing AD, while having one or two copies of the APOε-4 gene increases the likelihood (but does not guarantee) the individual will develop AD. Genetic mutations of the amyloid precursor protein—presenilin 1 or presenilin 2—are correlated with the development of early-onset AD (Table 2).3 Risk factors that may play a role in AD, but have not been causally confirmed, include oral health—especially bacterial infections and inflammation associated with uncontrolled periodontal diseases.
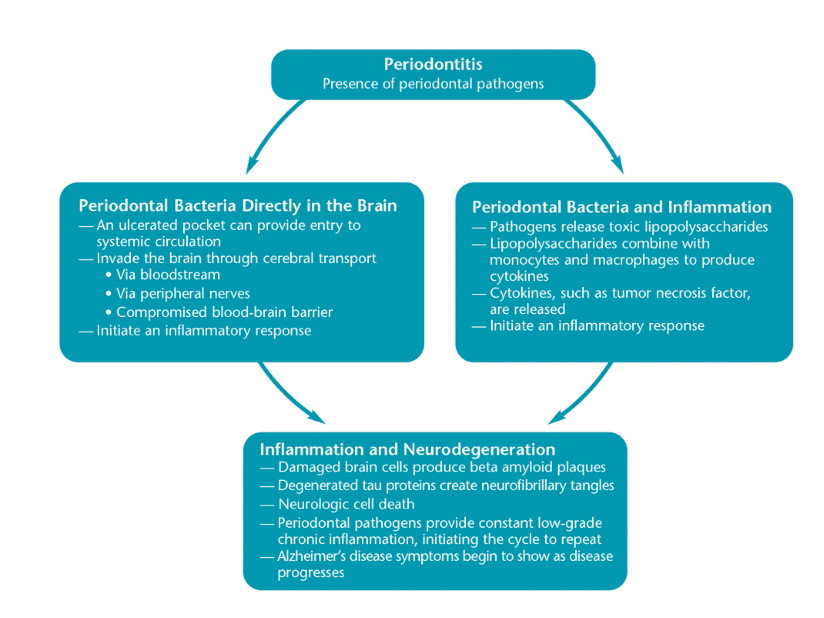
![Presence of Periodontitis in Alzheimer's Disease]() THE ROLE OF BACTERIA
THE ROLE OF BACTERIA
Research is underway to determine if certain oral health conditions, such as periodontal diseases, can initiate or progress the cognitive degeneration associated with AD. One theory hypothesizes that the beta amyloid plaques found in the brains of patients with AD can cause inflammation. The presence of inflammation can result in the blood-brain barrier becoming more permeable to aggressive periodontal bacteria, allowing more bacteria to enter the brain, which increases AD symptoms.8 Another theory suggests that invading periodontal spirochetes disrupt the brain’s normal defenses, allowing the bacteria to damage brain cells. The damaged brain cells produce beta amyloid protein as an adaptive response to the infection, which then accumulates into the beta amyloid plaques typical to AD.9 One study analyzed the frontal lobe cortex from 34 deceased patients, of which 16 had AD and 18 did not. Researchers detected oral Treponema (a spirochete bacteria involved in the progression of periodontal diseases) in 14 of the 16 patients with AD. Only four of the patients without AD had oral Treponema in the brain tissues.10 Another study analyzed the brain tissue of 20 patients, 10 with AD and 10 without, 12 hours post-mortem. The researchers found Porphyromonas gingivalis in four of the 10 brain samples of patients with AD and none in the samples without AD.9 A literature review found that spirochetes in the brain (from periodontal sources) had been detected with eight times higher frequency in subjects with AD compared to those without. The presence of spirochetes may create a continuous infection and inflammatory process that leads to the neuronal degeneration seen in AD.8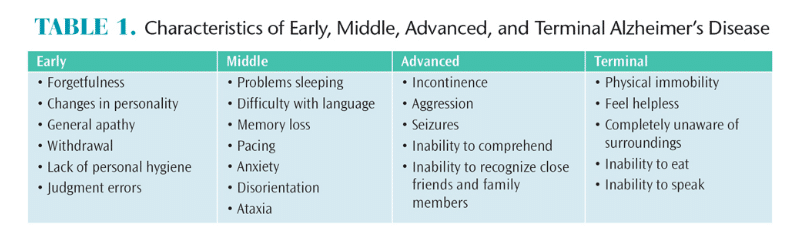
SYSTEMIC INFLAMMATION
Along with specific periodontal bacteria, systemic inflammation could be a risk factor for developing AD (Figure 1).11 The low-grade chronic inflammation caused by periodontal diseases can be a significant contributor to the systemic inflammation associated with chronic conditions, such as atherosclerosis, cardiovascular disease, and diabetes. As such, it may be that periodontal disease is a contributor to the development and progression of AD.12 During the immune response, B- and T-lymphocytes, macrophages, and plasma cells are transported to the inflamed area to control or eradicate periodontal pathogens, toxins, and other byproducts (eg, lipopolysaccharides). Lipopolysaccharides combine with the monocytes and macrophages to produce cytokines and inflammatory mediators such as interleukins (1α, 1β, and 6), prostaglandin E2, tumor necrosis factor (especially TNF-α), and matrix metalloproteinase—all of which contribute to inflammation and periodontal destruction.13 The ulcerated periodontal pocket may then become a gateway for bacteria to enter the circulatory system, contributing to systemic inflammation and potentially causing neuroinflammation and degeneration.12
Researchers are currently trying to determine whether pro-inflammatory molecules or serum levels of antibodies associated with periodontal diseases could be used to diagnose AD at its earliest stage.14 The National Health and Nutrition Examination Survey 1988-1994 was one of the first reports to suggest that systemic inflammatory markers could be linked to AD.15 In this cross-sectional study, an association was identified between the serologic marker for P. gingivalis and poor cognitive test performance among older participants. Individuals with the highest P. gingivalis immunoglobulin G (IgG) serologic marker showed significant delay in verbal recall and increased difficulty with math equations involving subtraction when compared to individuals with fewer P. gingivalis IgG markers.15 In one longitudinal study, the serum antibodies for seven oral bacteria associated with periodontal diseases were analyzed in 158 individuals. Of the participants studied, 81 developed either mild cognitive impairment or AD. During serum antibody data collection, the 81 participants who developed impairments had significantly elevated antibody levels for the seven periodontal bacteria years before they showed signs of cognitive deficits.16 Other studies aimed at analyzing the serum levels of TNF-α associated with chronic periodontal disease and inflammation have shown elevated serum levels among participants with AD compared to those without.17,18 The research proposes that TNF-α contributes to neurodegeneration by increasing inflammation locally and systemically, boosting deterioration of the blood-brain barrier, and triggering eventual cell death.19 Although it has not been determined that periodontal pathogens (or peripheral inflammation caused by the pathogens) can directly induce neural inflammation, a strong association has been found between periodontal diseases and systemic inflammation, which, in turn, is strongly correlated with dementia.14,20 Even without a definitive causal relationship, dental hygienists should educate patients on the importance of the oral-systemic link and be prepared to work with patients who have AD to maintain or improve oral health.
ORAL HEALTH
There are many oral health considerations for individuals with AD. In general, they have higher plaque scores, more decay, and fewer teeth than the general population. This decline in oral health can be attributed to the fact that individuals with AD are less able to perform adequate oral hygiene on a regular basis. Combined with loss of motor skills and softer food choices as chewing becomes more difficult, dental plaque control in patients with AD is difficult.21 The medications patients take to control AD symptoms can also negatively affect oral health. Xerostomia is a side effect of many drugs, and gingival hyperplasia can occur if the patient is taking anticonvulsants.3 Both medication associated disorders can exacerbate already poor oral health conditions.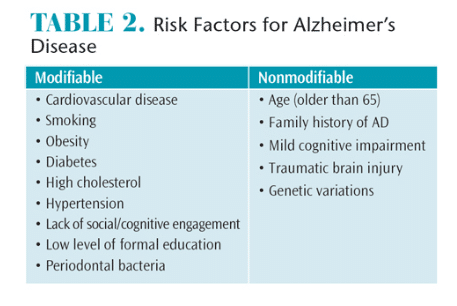
The primary dental hygiene goals for patients with AD are to preserve oral health and function and ensure comfort. Dental hygienists need to plan treatment keeping in mind that patients will most likely experience a dramatic decline in mental status and self-care as time progresses.6 Patients with AD should carry out their own care for as long as they can with the supervision of their caregiver. Using an electric toothbrush or a toothbrush with a modified handle is recommended to improve self-care. Individuals with AD should be involved in determining their course of treatment for as long as possible.3 Establishing a dental home early in the progression of AD can help patients feel more at ease in the familiar surroundings.6 Dental visits can be distressing, but individuals who have received routine dental care for a majority of their lives do much better with dental treatment throughout the course of the disease.3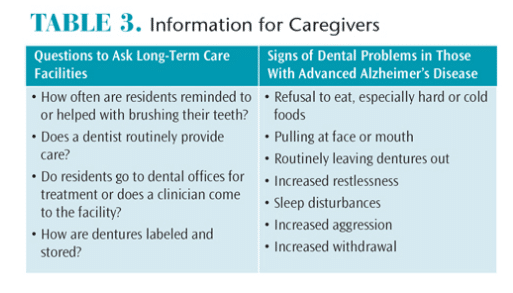
Intervals for continuing care appointments should be every 3 months, with fluoride varnish applied at each appointment to help maintain patients’ oral health status and prevent disease progression. Dental hygienists should perform intra- and extraoral exams at every appointment to look for sores or other problems patients may be unable to self-report.6 Dental hygienists should deliver instructions and phrase questions in short, easy-to-understand sentences. Patients should be given ample time to answer and communicate. Reminder cards for appointments as well as follow-up calls are a good way to ensure patients don’t forget appointments. Patients with AD who are edentulous should still have yearly appointments to check the fit of dentures and examine oral tissues. When patients can no longer handle the stress of dental appointments in the office, dental hygienists should consider visiting them in their homes or long-term care facilities (LTCFs) if state regulations permit.3 There may come a time when patients become combative and unaware of their surroundings. At this point, clinicians can use mouth props, sedation, or physical restraints to provide care. If the dental team does not feel comfortable using such devices, referral to a specialist may be necessary.3,6
Caregivers need to be involved early with dental therapies so they will be prepared to provide oral health care and make treatment decisions.6 Caregivers may find it is easier to brush teeth from a standing position behind the seated person they are helping. If the patient is living in an LTCF, the caregiver should be aware of the facility’s dental policies and procedures. As the disease progresses, patients with AD may lose the ability to communicate the presence of dental problems or discomfort. Dental hygienists should teach caregivers which behaviors indicate a patient is experiencing problems (Table 3).3
As AD advances, legal competency issues may arise. Legal competency is defined as the mental and cognitive capabilities needed to legally execute a rational act.3 In order to preserve a person’s basic right to autonomy, most legal competency matters are settled in a court of law where the individual is considered competent unless clear and convincing proof is presented.21 If a patient begins to show signs of AD, dental professionals should refer the patient to his or her primary care physician for evaluation and a definitive diagnosis.6 Once a diagnosis is made, individuals with AD should determine who they would like to serve as thier surrogate decision maker or guardian and write directives for care should they become unable to make those decisions.3
CONCLUSION
Dental hygienists can be an important resource for patients with AD and their caregivers by providing care and education through all phases of the disease. Education may include informing patients and caregivers about the possible influence of periodontal bacteria and inflammation on the development and progression of AD. While a direct link has not been causally established, there is clear association between uncontrolled periodontal bacteria, systemic inflammation, and neurodegenerative diseases. As the population ages, dental hygienists will be ready to meet the growing needs of older adults and continue to provide the highest level of preventive dental therapy.
REFERENCES
- Centers for Disease Control and Prevention.Alzheimer’s Disease. Available at: cdc.gov/aging/aginginfo/alzheimers.htm. Accessed October 14, 2015.
- Alzheimer’s Association. 2015 Alzheimer’s DiseaseFacts and Figures. Available at: alz.org/facts/overview.asp. Accessed October 14, 2015.
- Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11:332–384.
- Alzheimer’s Association. Alzheimer’s Disease—Pathological Substrate. Available at: alz.org/professionals_and_researchers_13519.asp. Accessed October 14, 2015.
- Brion JP. Neurofibrillary tangles and Alzheimer’s disease. Eur Neurol. 1998;40:130–140.
- Wilkins E, Wyche C. Clinical Practice of the Dental Hygienist. 11th ed. Philadelphia: Wolters KluwerHealth/Lippincott Williams & Wilkins; 2013.
- National Institute of Health. Genetics homereference. Available at: ghr.nlm.nih.gov/gene/APOE.Accessed October 14, 2015.
- Miklossy J. Alzheimer’s disease—a neurospirochetosis.Analysis of the evidence following Koch’s and Jill’s criteria. J Neuroinflammaion. 2011;8:90.
- Poole S, Singhrao SK, Kesavalu L, Curtis MA, Crean SJ.Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’sdisease brain tissue. J Alzheimers Dis. 2013;36:665–677.
- Riviere GR, Riviere KH, Smith KS. Molecular andimmunological evidence of oral Treponemain the human brain and their association with Alzheimer’s.Oral MicrobiolImmunol. 2002;17:113-–118.
- Perry HV, Newman TA, Cunningham C. The impact ofsystemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003; 4:103–112.
- Wu Z, Nakanishi, H. Connection betweenperiodontitis and Alzheimer’s disease: possible roles of microglia and leptomeningeal cells. J Pharmacol Sci. 2014;126:8–13.
- Darby ML, Walsh MM. In: Bowen DM, ed. DentalHygiene Theory and Practice . 4th ed. St. Louis: ElsevierInc; 2015:329.
- Cerajewska TL, Davies M, West NX. Periodontitis: apotential risk factor for Alzheimer’s disease. Brit Dent J. 2015;218:29–34.
- Noble JM, Borrell, LN, Papapanou PN, Elkind MSV,Scarmeas N, Wright CB. Periodontitis is associated with cognitive impairment among older adults: analysis of NHANES-III. J NeurolNeurosurg Psychiatry. 209;80:1206–1211.
- Stein PS, Steffen MJ, Smith C, et al. Serumantibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dement. 2012:8:196–203.
- Kamer AR, Craig RG, Pirraglia E, et al. TNF-cx andantibodies to periodontal bacteria discriminate between Alzheimer’s disease patients and normal subjects. J Neuroimmunol. 2009;216:92–97.
- Farhad SZ, Amini S, Khalilian A, et al. The effects ofchronic periodontitis on serum levels of tumor necrosis factor- alpha in Alzheimer disease. Dent Res J. 2014;11:549–552.
- Gurav AN. Alzheimer’s disease and periodontitis—an elusive link. Rev Assoc Med Bras. 2014;60:173–180. Watts A, Crimmins EM, Gatz M. Inflammation as apotential mediator for the association between periodontal disease and Alzheimer’s disease. Neuropsychiatr Dis Treat. 2008;4:24–29.
- Cicciu M, Matacena G, Signorino F, Brugaletta A,Cicciu A, Bramanti E. Relationship between oral health and its impact on the quality life of Alzheimer’s diseasepatients: A supportive care trial. Int J Clin Exp Med. 2013;6:766–772.
From Dimensions of Dental Hygiene. November 2015;13(11):64–67.