Advanced Strategies to Tackle Dental Caries
Explore cutting-edge preventive therapies and personalized interventions for high-risk dental caries patients.
This course was published in the March 2024 issue and expires March 2027. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
AGD Subject Code: 430
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Describe the progressive nature of caries in patients at high and extreme risk.
- Discuss adjunct prevention techniques that can maximize a patient’s oral care at home.
- Identify adjunct preventive therapies that are used in the professional dental setting.
As dental caries is complex and multifactorial, there is no simple cause-and-effect pathway. Dental caries arises from the concerted action of many genes; environmental factors; and risk-conferring behaviors.1 Oral health professionals can suggest adjunct preventive therapies to help mitigate caries’ deleterious effects. Two concepts for the science of prevention are the ecological plaque hypothesis and the Stephan curve.
The first is the idea is that dental caries is the result of a shift from homeostasis of the oral microflora to more pathogenic species caused by ecological stress. Thus the “ecology” of the oral cavity favors microflora that can produce and tolerate more acidic environments. Hence, prevention therapies should be directed at shifting the ecology back from dysbiosis toward homeostasis.
The second concept is from 1940 when Stephan showed that demineralization of enamel occurs at a pH < 5.5.2 Thus, prevention therapies may also attempt to counter the pH lowering ability of plaque. These broad concepts have been incorporated into a philosophy known as “caries management by risk assessment” (CAMBRA) in which individuals receive a caries risk assessment and personalized prevention treatment plan based on risk status.3
Oral health professionals are charged with creating a prevention plan for patients who fall into “high” and “extreme” caries risk categories (Figure 1). In high- and extreme-risk patients, caries progression cannot be controlled by conventional fluoride therapy and restorative treatment.
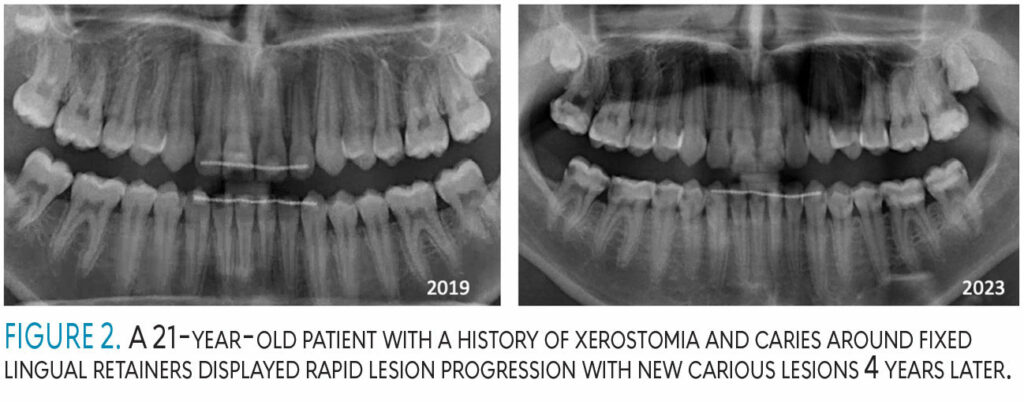
All clinical studies on such patients clearly show major caries progression in spite of combined fluoride and restorative therapy.3,4 Individuals experiencing significant caries progression will need a combination of therapies and active surveillance to determine if they are responding, or if caries continues to progress (Figure 2).
Individualized prevention plans should include behavior modification for diet and oral hygiene practices as well as strategies to address barriers faced by the patient as compliance is required for successful outcomes. Motivational interviewing is a method for encouraging people to make behavioral changes to improve health.5 Providing patients with a menu of possible options may help them commit to one or two preventive therapies.
Boosting Oral Hygiene
At-home adjunct therapies are available to help patients maximize their current hygiene practices. For example, while rinsing with water after brushing appears to be a cultural norm, multiple studies conclude that rinsing after brushing can reduce the benefit of fluoride toothpaste.6 Additionally, plaque removal by electric toothbrushes has been shown to be superior to that of manual toothbrushes.7,8 Strong evidence shows that daily use of fluoride toothpaste prevents dental caries in a dose-response relationship, with clinical trials demonstrating that high fluoride dentifrices (5,000 ppm) offer more protective action on caries than traditional toothpaste.9 Prescription-strength toothpaste is a first-line prevention step for patients with high caries risk.
To achieve maximal caries prevention while optimizing the effect of fluoride toothpaste, teeth should be brushed more than once a day with a power toothbrush for plaque removal7,8 using a 5,000 ppm dentifrice. Patients should floss before brushing to increase fluoride retention, avoid excessive rinsing with water after brushing,10 and abstain from eating or drinking for 2 hours post-brushing.
After maximizing the effects of hygiene routines, an antimicrobial rinse should be considered. A randomized clinical trial of adults who used chlorhexidine rinse daily for 1 week per month every month (in addition to brushing with fluoride toothpaste) for 2 years showed statistically significant reduction in cariogenic bacteria levels and a decrease in caries increment at 2 years.4 For extreme-risk patients, fluoride therapy must be supplemented by antibacterial therapy to reduce the bacterial challenge, modify the biofilm, and drive the oral ecology toward homeostasis.4
Another simple adjunct therapy is sugar-free gum (SFG). Chewing SFG has been shown to at least marginally reduce caries because the mechanical process of chewing stimulates saliva production, aids in plaque removal, and gum can be a carrier for bacteriostatic agents such as xylitol.11 Chewing SFG may also prohibit other high-risk behaviors such as the consumption of sugar-containing foods.
While the use of sugar alcohols as anti-cariogenic agents is controversial, in vitro studies suggest that xylitol is beneficial. Data regarding xylitol’s anticariogenicity are variable, and evidence of its antimicrobial effects to achieve caries prevention in adults and children is limited. Nevertheless, research indicates that xylitol as an adjunct therapy in caries prevention appears to be effective when added to SFG, because it has been shown to decrease Streptococcus mutans’ production of lactic acid, inhibit glucose uptake by S. mutans, and interfere with its attachment to the tooth surface.12,13
When compared to glucose, fructose, and sucrose, there is a distinct reduction in fermentation of xylitol, and xylitol does not lower the pH below 5.5. Xylitol consumption is regarded as generally safe when limited to 50 g/day for adults and 20 g/day for children.13 Studies suggest that in order for xylitol to be an effective agent in decreasing dental caries, it must be consumed three to five times a day for a total dose of 5 g to 10 g daily (frequencies less than three times a day were ineffective).12 To maximize SFG’s anticariogenic effect, patients could consider chewing SFG that contains at least 1 g of xylitol at least three times a day, especially after meals to stimulate saliva flow and improve oral clearance of food debris.
Xylitol’s negative side effect include stomach disturbances and diarrhea; however, these are not typically experienced unless the recommended daily dose is exceeded by four or five times.12
Patients with reduced salivary flow rate, whether due to medications or special health care needs, will experience more caries due to lower pH and buffering capacity. Increasing salivary flow rate yields remarkably increased levels of bicarbonate which buffers acid challenge and increases pH.14
Diagnosing dry mouth is not always straightforward, but a proper assessment of a patient’s medication list for xerostomic agents is imperative. For these patients, sodium bicarbonate (baking soda) mouthrinses have a significant alkalinizing effect on saliva, elevating the pH, promoting mineralization, and restraining overgrowth of aciduric bacteria in patients with dental caries. It also neutralizes acids, preventing erosion caused by both short-term exposure to strong acids, such as reflux and vomiting, and long-term exposure to weak acids, such as wine tasting.14
Sodium bicarbonate mouthrinse is an inexpensive and effective therapy for patients and can be prepared at home by dissolving ½ teaspoon of sodium bicarbonate in 1 cup of water. The unused mouthrinse should be discarded daily because tap water is not sterile. Instruct the patient to actively rinse after eating but to refrain from ingesting the solution, which may cause abdominal discomfort. Rinsing after snacks and meals will increase oral clearance and raise the pH of the oral cavity relatively immediately so that a patient does not spend long periods of time with the pH below 5.5.
Another at-home product for patients to consider is a sugar-free soft chew/candy containing arginine and calcium bicarbonate. Arginine is a natural component of healthy saliva, and microbes can metabolize arginine, producing the important by-product — ammonia — which drives salivary pH towards a neutral 7.15 The premise behind the chew is maintaining the oral environment above the critical demineralization point of 5.5, and, over time, this shift to a higher pH would select for more beneficial, pH-raising bacteria, establishing a healthy microbiome.
The bicarbonate in the product offers immediate buffering; calcium is available for remineralization of enamel; and arginine is offered as a substrate for ammonia production for sustained elevated pH. Promising studies have shown significant inhibition of caries progression.15 However, this therapy may be cost-prohibitive for some patients; it is considered a food product and would not be eligible for insurance coverage.
Professionally Applied Products
In conjunction with behavior modifications at home, several professionally applied products may be beneficial for patients. As children become adults, many oral health professionals stop providing professional fluoride applications likely due to the lack of third-party reimbursement. However, fluoride’s anti-caries efficacy is well-established, and a professional fluoride application should always follow a dental prophylaxis for high-risk patients. Additionally, abrasive polishing removes microns of enamel that are rich in fluoride and some research shows that selective polishing for stain removal is preferred.16
Fluoride varnish shows a substantial caries-inhibiting effect in trials in children.17 Promising work has been published on the combination of varnish with the antimicrobial agent, povidone iodine (PVP-I), which has been shown to significantly reduce the levels of S. mutans in plaque and saliva.18 Several trials in children have evaluated the caries inhibiting effects of applying PVP-I just prior to a fluoride varnish application three to six times per year.19,20 The technique is safe and quick. It includes swabbing 10% PVP-I on the teeth, allowing it to dry for 1 minute, and then placing varnish.
Silver diamine fluoride (SDF) offers benefits in certain clinical situations, and numerous systematic reviews substantiate its efficacy for both arresting and preventing new, carious lesions.21 SDF combines the antibacterial effects of silver and the remineralizing effects of fluoride.
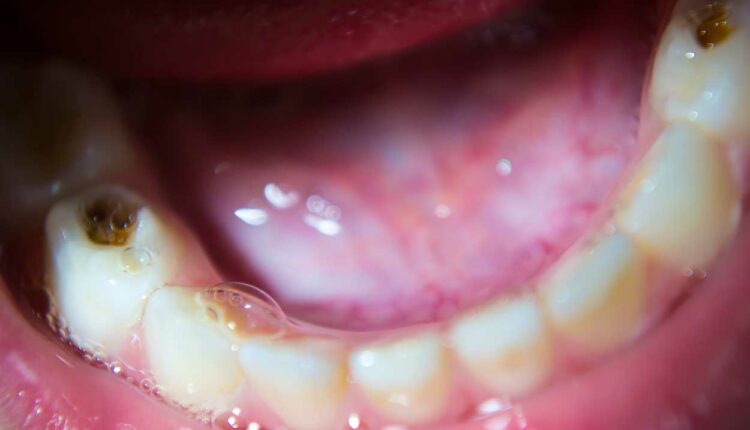
 SDF is affordable, easy to apply, and safe; however, it does stain the lesion and any demineralized tooth enamel dark black. While it can certainly be applied to cavitated lesions, SDF can also be used for demineralized cervical lesions in posterior molars that might not be esthetically visible (Figure 3) and/or for interproximal lesions that are decalcified but not cavitated.
SDF is affordable, easy to apply, and safe; however, it does stain the lesion and any demineralized tooth enamel dark black. While it can certainly be applied to cavitated lesions, SDF can also be used for demineralized cervical lesions in posterior molars that might not be esthetically visible (Figure 3) and/or for interproximal lesions that are decalcified but not cavitated.
An additional option for interproximal enamel lesions is the interproximal sealant and/or resin infiltration. A wealth of evidence supports sealants’ significant caries-preventive effect on fissure caries.22 Additionally, sealing noncavitated interproximal lesions is more effective than fluoride varnish or flossing instructions in preventing lesion progression.23 Studies have shown that lesions may be treated with either resin infiltration or with etching and placing traditional resin sealant if the clinician has direct access to the interproximal lesion (eg, the anterior tooth has exfoliated, the adjacent tooth has been prepared for a proximal restoration, or the clinician has placed an orthodontic separator).
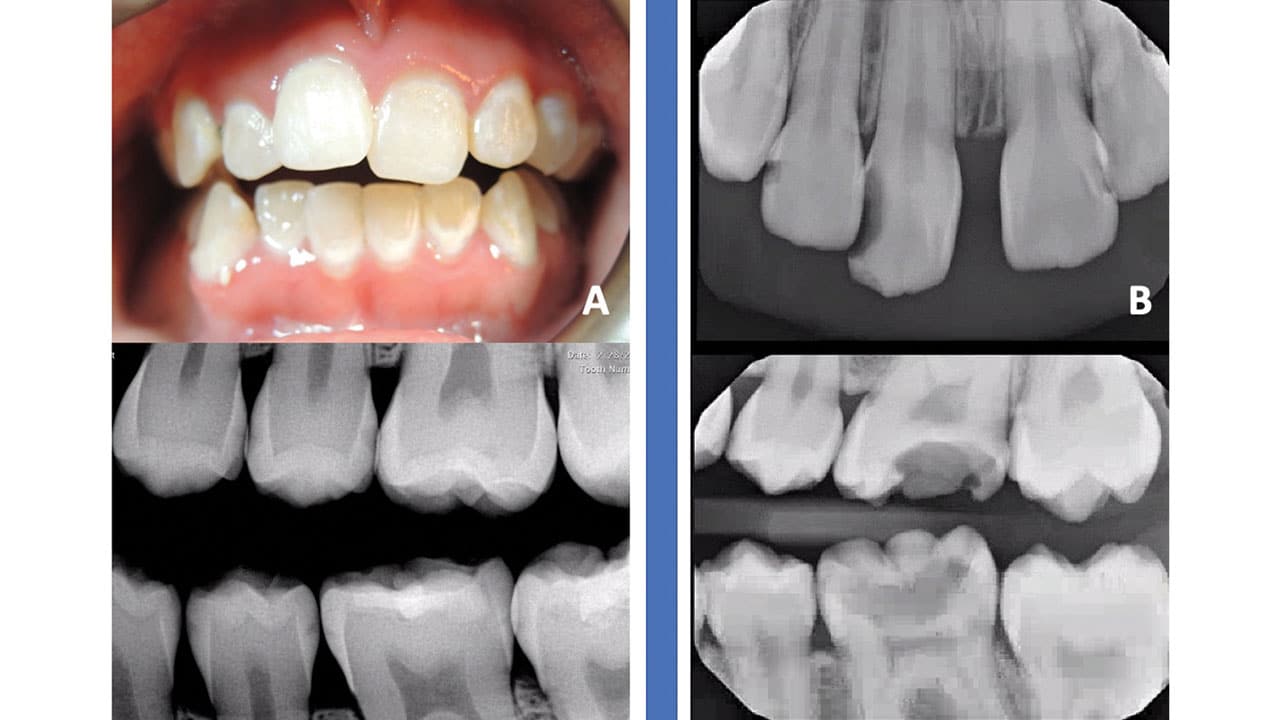
Another professionally applied agent for enamel regeneration contains the self-assembling peptide (SAP) P11-4. SAP was developed as a biomimetic approach for enamel regeneration for patients such as shown in Figure 1A (page 42). P11-4 is engineered in such a way that it spontaneously self-assembles in response to environmental triggers into a scaffold that is similar to the enamel matrix, facilitating remineralization.
In vitro studies and short-term in vivo studies show promising results of carious lesion regression (ie, improvement in demineralization).24 Two clinical studies, one of initial carious lesions on first permanent molars and another of white spot lesions after orthodontics, showed that teeth treated with SAP P11-4 exhibited significantly greater remineralization, lesion regression, and inactivation of carious lesions than no treatment or fluoride varnish alone 6 months later, particularly in combination with fluoride varnish.24
Long-term studies are needed to substantiate the findings, but short-term studies show superior effects of SAP treatment. The treatment is noninvasive, requires professional cleaning and etching of the tooth to remove organic and inorganic deposits, and then applying the solution and allowing it to diffuse into the tooth for 5 minutes. Third party payers may not yet reimburse for remineralization procedures, but the technique is feasible to implement with low resource utilization.
Conclusion
While restoration of the dentition is necessary for function and esthetics, patients should be educated that restoration alone does not prevent future caries. Dental caries is a chronic disease. Patients who understand the chronic nature of their disease and select self-management goals are more likely to make sustainable behavior changes.3 Extreme- and high-risk patients are not condemned to recare visits that continually end with news of a new cavity. Evidence suggests that combining preventive therapeutics and repeated application of anti-caries agents can reduce tooth decay among high-risk patients engaged in regular dental care.25
Routine diet counseling and brushing and flossing advice, while necessary, are unlikely to prevent caries progression in extreme-caries-risk patients (Figure 2, page 43). These patients will benefit from multimodal preventive therapies and require frequent monitoring such as 3-month recare appointments or reapplication of professional products.
While more frequent dental visits may help with keeping patients engaged in their own oral care, it may also require out-of-pocket expenses. Oral health professionals may consider any of the above options for patients and may implement them considering the costs and resources involved and preferences of the patient population. Robust research shows that beneficially altering the caries balance is necessary for the management of dental caries in all patients, and clinicians have a variety of therapeutics to consider as adjuncts to basic hygiene instructions.
References
- Fejerskov O. Changing paradigms in concepts on dental caries: consequences for oral health care. Caries Res. 2004;38:182–191.
- Stephan R. Changes in hydrogen-ion concentration on tooth surfaces and in carious lesions. J Am Dent Assoc. 1940;27:718–723.
- Featherstone JDB, Crystal YO, Alston P, et al. Evidence-based caries management for all ages-practical guidelines. Front Oral Health. 2021;2:657518.
- Featherstone JDB, Chaffee BW. The evidence for caries management by risk assessment (CAMBRA). Adv Dent Res. 2018;29:9-14.
- Lundahl B, Moleni T, Burke BL, et al. Motivational interviewing in medical care settings: a systematic review and meta-analysis of randomized controlled trials. Patient Educ Couns. 2013;93:157–168.
- Pitts N, Duckworth RM, Marsh P, Mutti B, Parnell C, Zero D. Post-brushing rinsing for the control of dental caries: exploration of the available evidence to establish what advice we should give our patients. Br Dent J. 2012;212:315–320.
- Grender J, Williams K, Walters P, Klukowska M, Reick H. Plaque removal efficacy of oscillating-rotating power toothbrushes: review of six comparative clinical trials. Am J Dent. 2013;26:68–74.
- Williams K, Ferrante A, Dockter K, Haun J, Biesbrock AR, Bartizek RD. One- and 3-minute plaque removal by a battery-powered versus a manual toothbrush. J Periodontol. 2004;75:1107–1013.
- Ekstrand KR, Poulsen JE, Hede B, Twetman S, Qvist V, Ellwood RP. A randomized clinical trial of the anti-caries efficacy of 5,000 compared to 1,450 ppm fluoridated toothpaste on root caries lesions in elderly disabled nursing home residents. Caries Res. 2013;47:391–398.
- Oral Health Foundation. “Spit Don’t Rinse” for Better Oral Health. Available at: dentalhealth.org/news/spit-dont-rinse-for-better-oral-health. Accessed September 6, 2023.
- Newton JT, Awojobi O, Nasseripour M, et al. A systematic review and meta-analysis of the role of sugar-free chewing gum in dental caries. JDR Clin Trans Res. 2020;5:214-23.
- ALHumaid J, Bamashmous M. Meta-analysis on the effectiveness of xylitol in caries prevention. J Int Soc Prev Community Dent. 2022;12:133–138.
- Janket SJ, Benwait J, Isaac P, Ackerson LK, Meurman JH. Oral and systemic effects of xylitol consumption. Caries Res. 2019;53:491–501.
- Walsh LJ. Preventive dentistry for the general dental practitioner. Aust Dent J. 2000;45:76–82.
- Zaura E, Twetman S. Critical appraisal of oral pre- and probiotics for caries prevention and care. Caries Res. 2019;53:514–526.
- Gibson TJ, Nash DA. Practice patterns of board-certified pediatric dentists: frequency and method of cleaning children’s teeth. Pediatr Dent. 2004;26:17–22.
- Marinho VC, Worthington HV, Walsh T, Clarkson JE. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2013:CD002279.
- Milgrom P, Tut O, Rothen M, Mancl L, Gallen M, Tanzer JM. Addition of povidone-iodine to fluoride varnish for dental caries: a randomized clinical trial. JDR Clin Trans Res. 2021;6:195–204.
- Amin MS, Harrison RL, Benton TS, Roberts M, Weinstein P. Effect of povidone-iodine on Streptococcus mutans in children with extensive dental caries. Pediatr Dent. 2004;26:5-10.
- Lopez L, Berkowitz R, Spiekerman C, Weinstein P. Topical antimicrobial therapy in the prevention of early childhood caries: a follow-up report. Pediatr Dent. 2002;24:204–206.
- Crystal YO, Niederman R. Evidence-based dentistry update on silver diamine fluoride. Dent Clin North Am. 2019;63:45-68.
- Wright JT, Crall JJ, Fontana M, et al. Evidence-based clinical practice guideline for the use of pit-and-fissure sealants: a report of the American Dental Association and the American Academy of Pediatric Dentistry. J Am Dent Assoc. 2016;147:672–682.
- Chen Y, Chen D, Lin H. Infiltration and sealing for managing non-cavitated proximal lesions: a systematic review and meta-analysis. BMC Oral Health. 2021;21:13.
- Aparna B, Yashoda R, Puranik MP. Remineralization of early enamel caries lesions using self-assembling peptides P(11)-4: Systematic review and meta-analysis. J Oral Biol Craniofac Res. 2022;12:324–331.
- Chaffee BW, Cheng J, Featherstone JD. Non-operative anti-caries agents and dental caries increment among adults at high caries risk: a retrospective cohort study. BMC Oral Health. 2015;15:111.
From Dimensions of Dental Hygiene. March 2024; 22(2):42-45