
Emerging Discoveries on the Pathogenicity of Porphyromonas Gingivalis
The role of this periodontal pathogen is multifactorial and has specific implications in neurodegenerative conditions.
This course was published in the October 2023 issue and expires October 2026. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
AGD Subject Code: 490
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the role of Porphyromonas gingivalis in the development and progression of periodontal diseases.
- Identify the emerging evidence of P. gingivalis‘ impact on neurodegenerative diseases.
- Note the connections between P. gingivalis, its virulence factors, and their effects on the blood-brain barrier, iron metabolism, and central nervous system homeostasis.
A major player in the initiation and progression of periodontal diseases, Porphyromonas gingivalis is a powerful manipulator of its own environment and commensurate bacteria, the host’s immune response and inflammation levels, and nutrients’ availability to sustain its spread within and outside the oral cavity.1–3 The bacterium’s unique features, virulence factors, role in the microbiome, and connections with systemic inflammation and diseases have long been studied.4,5 Yet, emerging evidence continues to shed light on its exceptional role as a periodontal pathogen with broad systemic influence.
The effects of P. gingivalis on neurons and their supporting cells highlight the distinctive features of this pathogen and underscore the role of dental health professionals in preventing and treating periodontal diseases as a way to help patients maintain their overall systemic health.
Research is ongoing regarding the role of various microbes in neurodegenerative diseases.6–9 In fact, it has been suggested that Alzheimer disease (AD) may be viewed as a “non-transmissible polymicrobial infection of the brain resulting from a dysbiotic host microbiome.”9 Remarkably, the synergistic relationship between P. gingivalis and the Actinomyces species in the oral cavity also repeats in the AD brain model, highlighting their interdependence.9 Treponema species, Aggregatibacter actinomycetemcomitans, Fusobacterium nucleatum, and Prevotella intermedia may also be involved in the pathogenesis of AD.10,11
As a polymicrobial disease, bacteremia associated with periodontal diseases consists of various species besides P. gingivalis, inviting further investigation into bacteria and their virulence factors’ penetration through the blood-brain barrier (BBB).12 Likewise, the hypothesized effect of bacterial toxins and inflammatory mediators on microglia certainly merits further investigation. Manipulation of the innate immune system resulting in the persistent activation of microglia also leads to excessive synaptic pruning and loss of neuronal connections.9
Some analyses of clinical studies exploring the causative connections between periodontal diseases and AD have suggested a 10-year timeframe from the establishment of chronic periodontitis to it becoming a risk factor for sporadic AD.10,11,13 Tooth loss due to periodontal diseases is estimated to double the risk of AD, with a loss of up to nine teeth having the highest risk of contributing to late-onset AD.11,14
While these findings are alarming, the preventability and treatment options for PD offer hope to both patients and oral health professionals. The importance of at-home oral hygiene and professional therapies in eliminating or at least lessening oral focal infection cannot be overstated.9,11,13,15
Impact on Central Nervous System and Brain
Pathways to the central nervous system (CNS) for periodontal pathogens include: through the blood stream and penetration of the BBB by the microbes and their virulence factors and by traveling along the anatomically-proximal perivascular spaces and afferent nerves.6,16,17 Of these, the invasion via penetration of the weakened BBB demonstrates the effect of frequent low- and high-level bacteremia on the integrity of the BBB.
The neurovascular unit of the BBB is composed of endothelial cells, pericytes, astrocytes, and neurons.15 The microvascular endothelial cells of the BBB are uniquely equipped to minimize penetration due to their intracellular tight junctions with high electrical resistance, lack of transcellular pores, and the basement membrane shared with pericytes, which reduces pinocytic ability.12,15 The BBB undergoes age-related changes which may be further exacerbated by systemic diseases.15,17 On the other hand, the compromised “leaky” BBB contributes to further neurodegeneration by allowing circulating pathogens, cytokines, and pro-inflammatory factors, and toxins to enter the brain (Figure 1, page 34).15,17
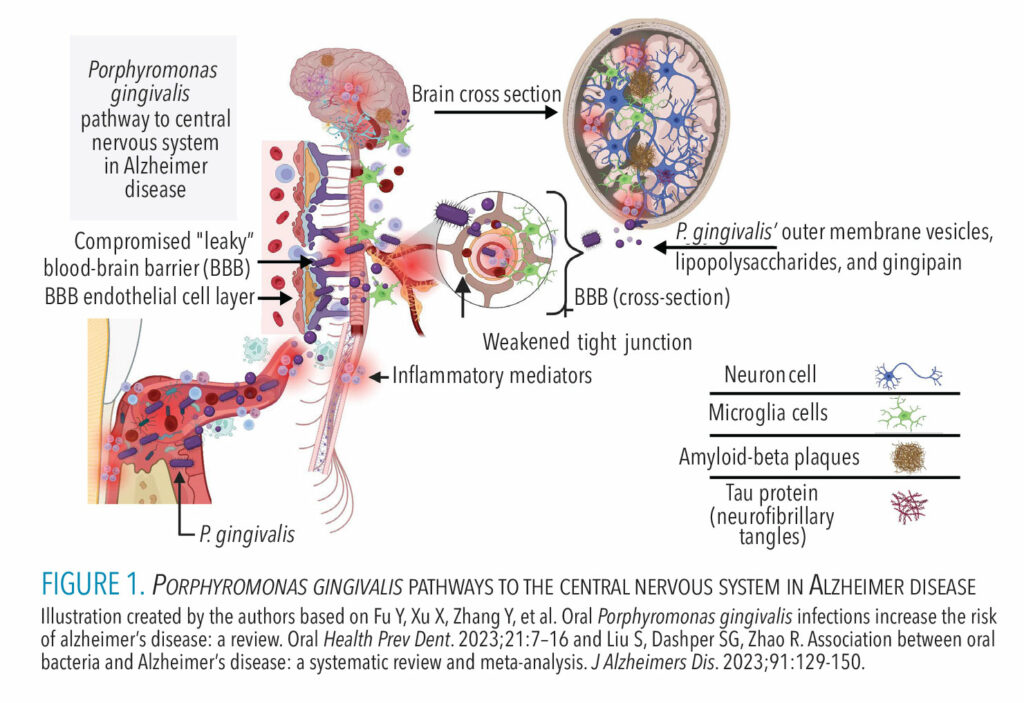 The pathogenesis of AD is multifactorial, involving a combination of risk factors, genetic predisposition, and age-related brain changes. The gene APOE ε4 allele is recognized as a susceptible genetic risk factor for AD.15,17 APOE ε4 allele causes an atypical heightened pro-inflammatory response of cytokines, which is also linked with the exacerbation and severity of periodontitis.18 Chronic inflammatory mediators are also associated with compromising the effectiveness of proteins responsible for maintaining the structural integrity of the BBB, permitting pathogens to pass. Furthermore, individuals with the APOE ε4 allele tend to exhibit an inflammatory phenotype, which is also associated with atherosclerosis and other cerebral and cardiovascular conditions.11
The pathogenesis of AD is multifactorial, involving a combination of risk factors, genetic predisposition, and age-related brain changes. The gene APOE ε4 allele is recognized as a susceptible genetic risk factor for AD.15,17 APOE ε4 allele causes an atypical heightened pro-inflammatory response of cytokines, which is also linked with the exacerbation and severity of periodontitis.18 Chronic inflammatory mediators are also associated with compromising the effectiveness of proteins responsible for maintaining the structural integrity of the BBB, permitting pathogens to pass. Furthermore, individuals with the APOE ε4 allele tend to exhibit an inflammatory phenotype, which is also associated with atherosclerosis and other cerebral and cardiovascular conditions.11
Gingipains are significant virulence factors produced by P. gingivalis. They are instrumental in evading cell death triggered by the host’s immune response and discharge of defense cells. Gingipains are responsible for 85% of P. gingivalis’ proteolytic activity. They are typically situated within the outer membrane vesicles on its cell surface. P. gingivalis is the only human bacterium able to produce gingipains.19
Highly aggressive gingipain variants have been detected in the brains of 90% of patients with AD.20 Their presence corresponds with the pathological breakdown findings of the ubiquitin-proteasome system and the occurrence of abnormal tau protein accumulations, one of the hallmark indicators of AD.20 Ubiquitin is a recognition marker for intracellular protein degradation.20,21 P. gingivalis gingipains can exert their influence on various cellular systems. In microvascular endothelial cells, they degrade the epithelial JAM-1 protein, leading to the induction of programmed cell death (apoptosis).15,22 Similarly, in lung epithelial cells, the gingipains provoke apoptosis.15
Although there are no reports of viable P. gingivalis being recovered from the human or animal brain tissue,6 a recent in vitro study demonstrated P. gingivalis’ ability to invade and endure within neurons, while simultaneously producing intraneuronal gingipains.20 This ability is thought to produce the neuronal dysfunction frequently observed in neurodegenerative disorders like AD.7,10,12
The entry of P. gingivalis or its virulence factors across the BBB instigates a sustained inflammatory activation. It induces AD-like characteristics. Moreover, microglia (specialized central nervous system macrophages) aid in preserving neuronal interconnections and synapse pruning, which supports healthy brain plasticity and the complex neural communication system of the brain.6 However, as microglia cells become chronically activated or dysregulated, they contribute to neuroinflammation and neuronal injury.6,15
Gingipains are situated on both the outer membrane and outer membrane vesicles of the P. gingivalis bacterium. Remarkably, the number of gingipains expressed on outer-membrane vesicles is three to five times higher than the number found on the P. gingivalis parent cell.6,23 Consequently, the presence of gingipains on outer-membrane vesicles enables P. gingivalis to trigger various aspects of AD pathology.24 Gingipains are able to cleave tau protein within specialized cells linked to the neuroblastoma cell. As a result, specific peptides released through this mechanism initiate the assembly of paired helical filaments associated with neurofibrillary tangles.6,15
The outer membrane vesicles of P. gingivalis survive and migrate through the body while most importantly averting degradation of its DNA and RNA, even after the internalization of host cells.23 This distinctive feature enables P. gingivalis to maintain essential genetic material and signaling molecules for continual cell-to-cell communication.
Due to their small size, adhesive properties, and proteolytic activities, outer membrane vesicles derived from P. gingivalis exhibit a tendency to disseminate within tissues.24 These features allow genetically intact outer membrane vesicles to circulate through the bloodstream to the endothelial cells of the CNS, causing disruptions in the BBB. Researchers also suggest that peripheral macrophages may engulf outer membrane vesicles and transport them to the brain.6,23,24
The lipopolysaccharide located on the outer membrane surface of P. gingivalis is a highly toxic component responsible for activating the host’s immune cell response.6,15,25 Dispersion of lipopolysaccharide is facilitated by P. gingivalis’ outer membrane vesicles through the type IX secretion system.15,24 This activation primarily affects monocytes and macrophages, triggering a series of events involving lysosomal enzymes, cytokines, reactive oxygen species, and nitric oxide. Consequently, these events lead to cellular damage, apoptosis, and inflammation.
Impairments in spatial learning and memory, as well as reduced passive avoidance learning, were observed in mice that underwent in vivo studies involving the injection of P. gingivalis-lipopolysaccharide.25 Additionally, the cortex and hippocampus exhibited activation of astrocytes and microglia, which was accompanied by an elevation in the levels of inflammatory cytokines.25 Post-mortem analysis of brain tissue samples of individuals with AD revealed considerable levels of the outer membrane vesicles and lipopolysaccharide virulent factors produced by P. gingivalis, providing insight into its the mechanism of action for permeating through the BBB.15
Recent reports confirmed reduced BBB integrity in rats following inoculation with P. gingivalis, and in a human endothelial cell BBB model following exposure to P. gingivalis and its lipopolysaccharide and outer membrane vesicles.12,15 Notably, both studies showed more significant effect on BBB permeability with higher levels of bacteria and its virulence factors. While low-level/low-frequency exposures also produced negative effects on the cells of BBB, the ability to recover its integrity was observed.12,15 Meanwhile, a higher bacterial and virulence factors load resulted in more significantly increased permeability.12
Investigation into the effect of P. gingivalis lipopolysaccharide on the BBB revealed that lipopolysaccharide has the capacity to induce oxidative stress, activate glial cells, and impair the integrity of tight junctions within the neurovascular unit in the central nervous system.15,19 The effects of lipopolysaccharide within the neurovascular unit contribute to the disruption of normal physiological processes.6,23
The invasive capabilities of P. gingivalis depend on the strain and the expression of fimbriae and gingipains in its outer membrane. The outer membrane vesicles of P. gingivalis are capable of vaster cellular degradation and incite innate immune responses, exceeding the magnitude of the response triggered by the bacteria alone. P. gingivalis also affects the dyshomeostasis of iron in the brain.6,26 The dysregulation of iron neuronal metabolism leads to a type of neuronal death distinct from apoptosis and necrosis.27 “Ferroptosis” is an iron-dependent programmed cell death pathway resulting in mitochondrial and cellular wall rupture due to iron overload and accumulation of a lethal level of lipid peroxides.27
Aging and inflammation disrupt the normal molecular mechanisms of iron regulation, leading to deposition of iron in the tissues.6,27 In AD, iron contributes to hyperphosphorylation of tau protein and dysfunction of both tau and amyloid precursor protein, accelerating senile plaque deposition and formation of neurofibrillary tangles.6,27 As iron cycles between its ferrous and ferric states, it is the ferric iron that accumulates in the amyloid-β (Aβ) plaques and neurofibrillary tangles.27 On the other hand, Aβ and tau can further disrupt iron metabolism, suggesting a positive feedback relationship in the AD pathogenesis.26,27 P. gingivalis has an absolute requirement for iron, whose availability to microbes is limited by the host’s innate nutritional immunity.4,26 P. gingivalis developed the means to overcome the body’s iron-limiting mechanisms.6,26 In fact, ~17% of the P. gingivalis surface proteome is devoted to heme capture, and the bacterium has the ability to modify its nutritional environment to obtain iron from its pathobionts.4,6,26 Locally, P. gingivalis satisfies its nutritional need for iron using hemoproteins in saliva, gingival crevicular fluid ,and erythrocytes. Once it reaches the bloodstream from a periodontal pocket, it acquires heme from circulating hemoglobin.26 In the brain, there are multiple mechanisms of iron acquisition that are currently under investigation, and the outcome is an increase in iron dysregulation already existing in the AD brain.
Conclusion
Investigation into the role of P. gingivalis in AD is ongoing. While the direct causative links have not yet been elucidated, the connection between periodontal diseases and systemic disease is undisputable. The role of dental professionals in preventing and treating oral disease as a focal infection is vital in preventing or reducing broader systemic effects.
References
- Hajishengallis G, Liang S, Payne MA, et al. A low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and the complement pathway. Cell Host Microbe. 2011;10:497–506.
- Mei F, Xie M, Huang X, et al. Porphyromonas gingivalis and its systemic impact: current status. Pathogens. 2020;9:944.
- Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30.
- Marsh I, Matthews A. The pathogenecity of Porphyromonas gingivalis. Dimensions of Dental Hygiene. 2022;20(10)34-37.
- Matthews A, Marsh I. The relationship between Porphyromonas gingivalis and systemic disease. Dimensions of Dental Hygiene. 2022;20(11)28–33. https://dimensionsofdentalhygiene.com/article/relationship-between-porphyromonas-gingivalis-systemic-disease/
- Liu S, Butler CA, Ayton S, Reynolds EC, Dashper SG. Porphyromonas gingivalis and the pathogenesis of Alzheime’s disease. Crit Rev Microbiol. 2023;0(0):1-11.
- Olsen I. Porphyromonas gingivalis-induced neuroinflammation in Alzheimer’s disease. Front Neurosci. 2021;15.
- Franciotti R, Pignatelli P, Carrarini C, et al. Exploring the connection between porphyromonas gingivalis and neurodegenerative diseases: a pilot quantitative study on the bacterium abundance in oral cavity and the amount of antibodies in serum. Biomolecules. 2021;11:845.
- Singhrao SK, Harding A. Is Alzheimer’s disease a polymicrobial host microbiome dysbiosis? Expert Rev Anti Infect Ther. 2020;18:275-277.
- Wu DT, Cho YW, Spalti MD, Bishara M, Nguyen TT. The link between periodontitis and Alzheimer’s disease — emerging clinical evidence. Dent Rev. 2023;3:100062.
- Singhrao SK, Olsen I. Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer’s disease. J Oral Microbiol. 2019;11:1563405.
- Lei S, Li J, Yu J, et al. Porphyromonas gingivalis bacteremia increases the permeability of the blood-brain barrier via the Mfsd2a/Caveolin-1 mediated transcytosis pathway. Int J Oral Sci. 2023;15:1-12.
- Olsen I. Can Porphyromonas gingivalis contribute to Alzheimer’s disease already at the stage of gingivitis? J Alzheimers Dis Rep. 2021;5:237-241.
- Dioguardi M, Crincoli V, Laino L, et al. The role of periodontitis and periodontal bacteria in the onset and progression of Alzheimer’s disease: a systematic review. J Clin Med. 2020;9:495.
- Pritchard AB, Fabian Z, Lawrence CL, Morton G, Crean S, Alder JE. An investigation into the effects of outer membrane vesicles and lipopolysaccharide of Porphyromonas gingivalis on blood-brain barrier integrity, permeability, and disruption of scaffolding proteins in a human in vitro model. J Alzheimers Dis. 2022;86:343-364.
- Olsen I, Singhrao SK. Can oral infection be a risk factor for Alzheimer’s disease? J Oral Microbiol. 2015;7:10.
- Kanagasingam S, Chukkapalli SS, Welbury R, Singhrao SK. Porphyromonas gingivalis is a strong risk factor for Alzheimer’s disease. J Alzheimers Dis Rep. 2020;4:501-511.
- Liccardo D, Marzano F, Carraturo F, et al. Potential bidirectional relationship between periodontitis and Alzheimer’s disease. Front Physiol. 2020;11:519866.
- Fu Y, Xu X, Zhang Y, et al. Oral Porphyromonas gingivalis infections increase the risk of Alzheimer’s disease: a review. Oral Health Prev Dent. 2023;21:7-16.
- Haditsch U, Roth T, Rodriguez L, et al. Alzheimer’s disease-like neurodegeneration in Porphyromonas gingivalis-infected neurons with persistent expression of active gingipains. J Alzheimers Dis. 2020;75:1361-1376.
- Liu S, Dashper SG, Zhao R. Association between oral bacteria and Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis. 2023;91:129-150.
- Lunar Silva I, Cascales E. Molecular strategies underlying Porphyromonas gingivalis virulence. J Mol Biol. 2021;433:166836.
- Okamura H, Hirota K, Yoshida K, et al. Outer membrane vesicles of Porphyromonas gingivalis: novel communication tool and strategy. Jpn Dent Sci Rev. 2021;57:138-146.
- Nara PL, Sindelar D, Penn MS, Potempa J, Griffin WST. Porphyromonas gingivalis outer membrane vesicles as the major driver of and explanation for neuropathogenesis, the cholinergic hypothesis, iron dyshomeostasis, and salivary lactoferrin in Alzheimer’s disease. J Alzheimers Dis. 2021;82:1417-1450.
- Zhang J, Yu C, Zhang X, et al. Porphyromonas gingivalis lipopolysaccharide induces cognitive dysfunction, mediated by neuronal inflammation via activation of the TLR4 signaling pathway in C57BL/䁴mice. J Neuroinflammation. 2018;15:37.
- Olsen I. Porphyromonas gingivalis may seek the Alzheimer’s disease brain to acquire iron from its surplus. J Alzheimers Dis Rep. 2021;5:79-86.
- Wang F, Wang J, Shen Y, Li H, Rausch WD, Huang X. Iron dyshomeostasis and ferroptosis: a new Alzheimer’s disease hypothesis? Front Aging Neurosci. 2022;14.
From Dimensions in Dental Hygiene. October 2023; 21(9):32-35



