The Complexities of Erosive Tooth Wear
The evolutionary roots and modern contributors to enamel erosion shed light on preventive strategies and clinical management.
This course was published in the April/May 2024 issue and expires May 2027. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
AGD Subject Code: 010
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the embryological processes that shape enamel structure and properties, contributing to its unique resistance to wear.
- Identify the diverse range of intrinsic and extrinsic causes of erosive tooth wear.
- List evidence-based recommendations to mitigate erosive tooth wear risks.
Properties of human enamel have been vastly explored from embryological, biomechanical, and functional viewpoints. Enamel, the hardest tissue in the body, is designed to withstand damage like no other biological construct.1 The incredible fatigue- and wear-resistant structure of human enamel is an evolutionary adaptation largely credited to the changes in the diet and lifestyle of the first humans.2
Both the thickness and hardness of enamel of our herbivorous relatives are inferior to humans with their omnivorous dietary habits.2 Enamel’s construction begins during fetal development. As each part of the tooth scaffolding gets built, the enamel layer assumes its shape and develops its properties predetermined by evolution.
The sophisticated process of enamel development, however, holds a significant shortcoming: enamel’s self-repair capabilities are minimal and fail to withstand continuous mechanical and acidic challenges of contemporary diet and human’s daily activities. Initial subclinical enamel loss turns into observable enamel deficiency patterns and can eventually result in significant tooth wear.3
Tooth Wear Patterns
Four noncarious tooth wear patterns have been recognized by dental science: abrasion, attrition, erosion, and abfraction.4,5 Tooth wear processes can be separated by both mechanical and chemical causative forces. The mechanical forces work through microfractures, grinding and chipping while the chemical forces dissolve hydroxyapatite crystals via acid exposure, thus, undermining the structure from within.3 Notably, the separation between the two forces is primarily theoretical. In reality, they interact by aiding and stimulating one another and result in cumulative tooth structure loss.6 As such, the sole attribution of enamel wear to mechanical forces does not explain the severity of lesions.7
The erosive component, an exposure of the structure to an acidic agent, is often present as the softening of the enamel shield is the frequently observed first step that opens the doors to destructive mechanical forces.8 Understanding individual processes contributing to tooth wear is valuable. Even more important is appreciating the common denominator and the most prolific contributor to tooth wear — dental erosion.9
Unless a severe episode of tooth erosion through exposure to a highly acidic substance occurs, tooth wear develops gradually and increases over time. The typical signs include subtle loss of normal occlusal and incisal morphology and surface texture, as well as thinning of the cervical periphery.8 Hypersensitivity may be a part of the initial set of signs and symptoms. The more advanced presentation includes dentin exposure and occlusal vertical dimension loss accompanied by the notable height discrepancy between existing restorations and unrestored teeth.8,10
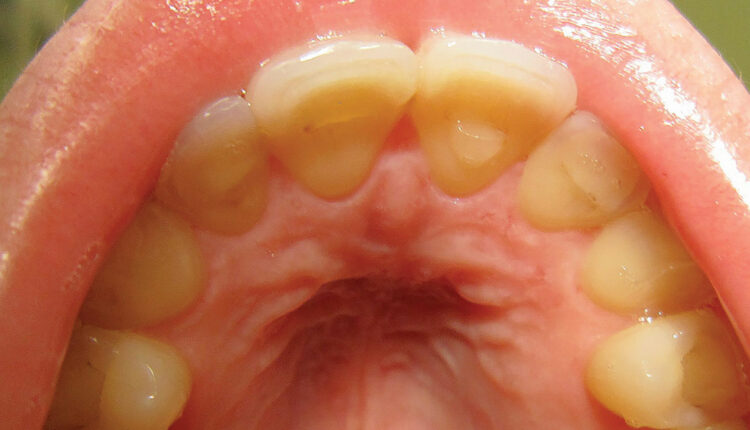
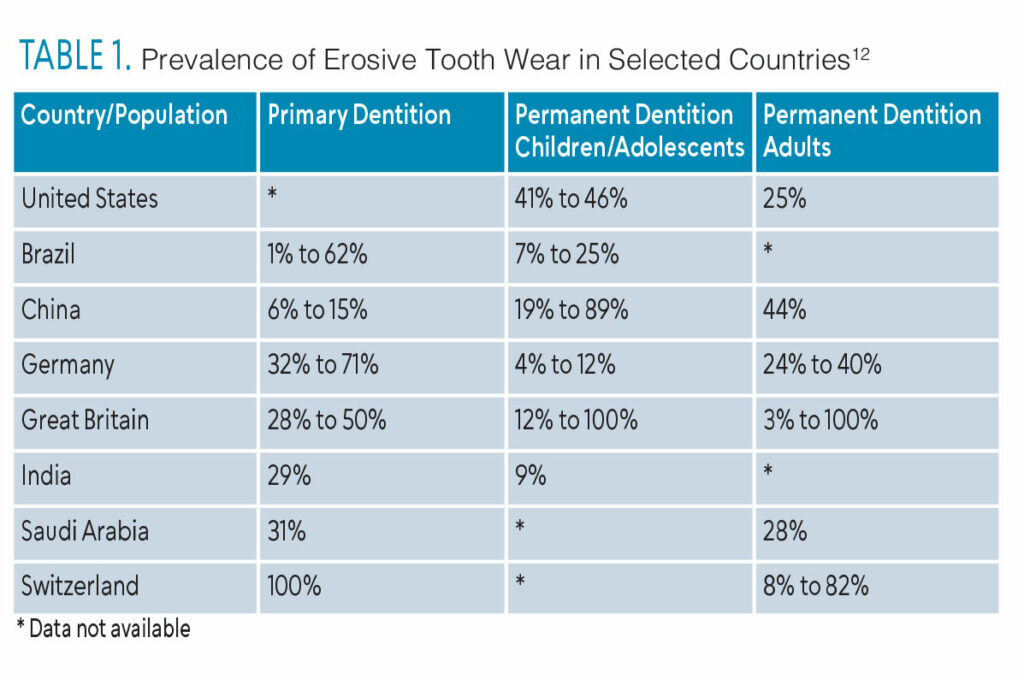 Even though time is a contributing factor, erosive tooth wear is far from an exclusive threat to older adults. The worldwide prevalence of pathological tooth wear is estimated to affect up to 50% of primary and permanent dentition.11 The available data on the worldwide prevalence of erosive wear are alarming for both children and adults (Table 1).12
Even though time is a contributing factor, erosive tooth wear is far from an exclusive threat to older adults. The worldwide prevalence of pathological tooth wear is estimated to affect up to 50% of primary and permanent dentition.11 The available data on the worldwide prevalence of erosive wear are alarming for both children and adults (Table 1).12
Erosion may be caused by both extrinsic and intrinsic acid sources and is not limited to any specific population.11 However, not every individual exposed to acids will develop erosion. The length, frequency, and pattern of erosive challenges affect the risk of tooth wear.4
Gastric Issues
Gastric juice is the single intrinsic source of acid profoundly associated with erosive tooth wear.12 In gastroesophageal reflux disease (GERD), gastric acid is introduced to an oral cavity with the reflux of stomach content. By some accounts, this condition affects up to a quarter of the world population, is common among children and adolescents, is frequently asymptomatic, and is often underdiagnosed.12,13
Issues with bulimia nervosa or disordered eating that involve frequent vomiting are another avenue for stomach acids to be introduced into the oral cavity and a significant risk factor for erosive tooth wear. Due to a variety of challenges (eg, stigma, lack of screening, etc), it is difficult to determine the prevalence of undiagnosed cases. The reported prevalence of eating disorder diagnoses in children is 3% and just under 1% in adults.14,15 However small, population groups affected by issues with disordered eating represent a significant number of observed erosive wear cases, with instances of up to 98% of occurrences for some of the disordered eating patterns.12
Food, Beverages, Medications, and Environment
Acidic drinks and food are the number one source of extrinsic acids implicated in erosive tooth wear. Studies show a significant relationship between acidic diet and tooth wear in both children and adults.16 The risk is influenced by intake frequency, the presence of carbonation, and the style of consumption.17 Alcohol content adds to the risk as well.8
Chewable or effervescent acidic drugs and vitamins, such as vitamin C, iron supplements, and asthma medications, may serve as potential risk factors.12 Many medications list xerostomia as side effect, which may also increase risk.
Wine tasters, swimmers, workers exposed to battery and galvanizing chemicals, and other industrial occupations are at a higher risk for erosion, especially if safeguards are not in place.18
Erosive Wear Indices
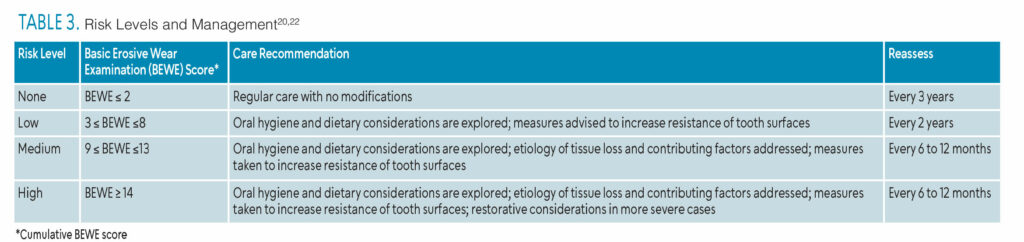
 Oral health professionals may want to consider incorporating erosion screening into their clinical practice. It can be challenging to consistently identify and classify the severity of noncarious tooth wear.19 As such, there is a need for a valid and reliable tool to measure and record tooth wear. The development of erosive lesions can be slow and subtle. thus, meticulous assessment and recordkeeping can trigger evidence-supported preventive steps, helping to avoid further damage. A few indices exist in oral health research (Table 2) with one that stands out for its practicality, validity, and reliability: basic erosive wear examination (BEWE).12,20–30
Oral health professionals may want to consider incorporating erosion screening into their clinical practice. It can be challenging to consistently identify and classify the severity of noncarious tooth wear.19 As such, there is a need for a valid and reliable tool to measure and record tooth wear. The development of erosive lesions can be slow and subtle. thus, meticulous assessment and recordkeeping can trigger evidence-supported preventive steps, helping to avoid further damage. A few indices exist in oral health research (Table 2) with one that stands out for its practicality, validity, and reliability: basic erosive wear examination (BEWE).12,20–30
The BEWE uses a number from zero to three to assess the most severely worn clinical surface per dental sextant. Zero indicates the absence of erosive tooth wear, one describes the initial loss of surface texture, two refers to the distinct effect with the tooth structure loss at less than 50% of the surface area, and three indicates the tissue loss of equal or more than 50% of the surface area.22 The sum of the scores, which may range between zero and 18, is recorded as the patient’s BEWE score.22
Risk Management
Once the BEWE score is established, it can be used to guide erosive wear risk management. Table 3 is an index that presents pathways for tooth wear management with levels from low to high.23 Further research on this subject is needed.22
When the signs of erosive tooth wear are identified, the patient’s medical and psychosocial history should be reviewed to identify risk factors. However, clinicians must remember that the early signs of erosive tooth wear are often difficult to recognize. Subtle changes in tooth anatomy and slight loss of normal surface appearance can be easily misconstrued as variations of normal and, therefore, overlooked.11 Hence, basic preventive strategies and recommendations should be included in the standard process of care. A dietary analysis should assist in discussing the acidity of the food and drinks as well as the consumption habits. Referrals should be made if signs of GERD, disordered eating, or other conditions associated with intraoral acid exposure are present. The following evidence-supported recommendations should be offered to patients who are at risk for developing erosive tooth wear:8,16,20,31
- Seek medical care to address etiological factors that are beyond individual control.
- Reduce the frequency of dietary acid consumption and switch to less acidic food and drink alternatives whenever possible.
- Continue practicing regular self-care to avoid caries and periodontal diseases.
- Use erosion-protective dentifrices and mouthrinses. Products containing metal ions, such as stannous fluoride, have been shown to significantly improve enamel’s resistance to early wear.20
Case Study
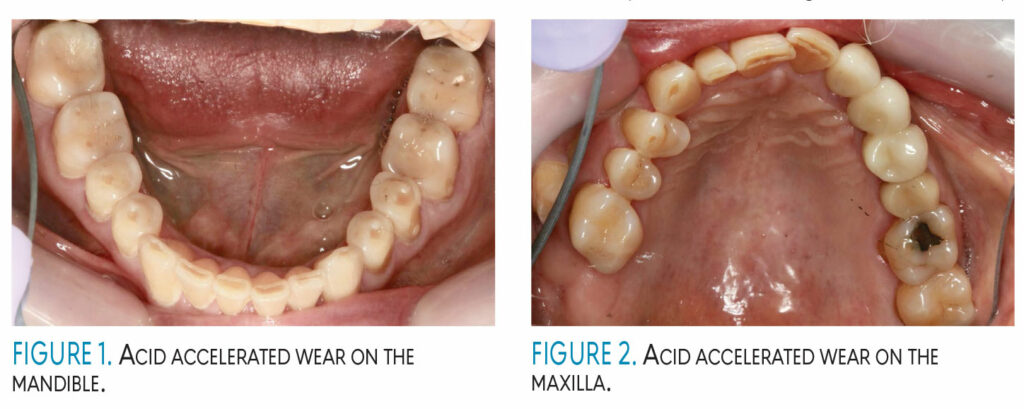
A 49-year-old white man in good health presented to the dental office for his 3-month periodontal maintenance appointment. He is not taking any prescription medications but reports consuming over-the-counter supplements for joint health. He receives regular dental and medical care.
His chief complaint was bad taste in the mouth in the morning and during exercise. Due to possible GERD, the patient was noted as having a mild systemic disease, but otherwise, no other significant medical findings were found and all vital signs were normal. He maintains a stringent exercise routine that includes running, skiing, and biking. The patient consumes a mostly healthy home-cooked diet that includes a significant amount of raw fruits and vegetables. He prefers plain carbonated water while exercising.
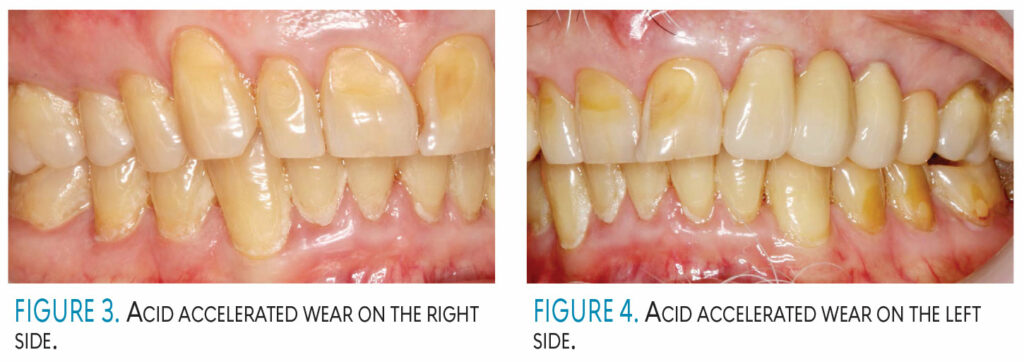
The patient’s clinical and radiographic findings can be seen in Figures 1 through 4. The patient was caries free with only light calculus and light to moderate biofilm. His previously diagnosed periodontal disease is well maintained.
The assessment showed a BEWE score of 13, which indicated a medium risk for erosive tooth wear. Patient reports using whitening toothpaste with sodium fluoride and a power toothbrush. He does not replace the brush head until the bristle wear is obvious.
The patient reported a bad taste when he wakes up and while exercising. Further assessment identified signs of intraoral intrinsic acid exposure. The patient is referred to medical care for evaluation of GERD.
Dental care modifications should include semi-supine position and appointments scheduled later in the day. An athletic mouthguard is advised but should be used cautiously if the reflux is not controlled, as it can serve as a reservoir for acid and increase erosive wear over time.
The dental hygienist recommended the use of a toothpaste with stannous fluoride and an oscillating-rotation power brush with a built-in pressure sensor. Additionally, he was advised to follow the brushing technique appropriate for the power toothbrush and replace the brush head every 3 months.
Other suggestions included the use of a drinking straw when carbonated water is consumed, continued periodontal maintenance, and undergoing an evaluation for erosive wear every 6 months.
Conclusion
Understanding the intricate processes of enamel development and the multifaceted causes of tooth wear, particularly erosion, is crucial in modern dentistry. With a growing prevalence of erosive tooth wear globally, comprehensive risk assessment and preventive strategies are imperative to mitigate its impact on oral health.
References
- Vaissier Welborn V. Enamel synthesis explained. Proc Natl Acad Sci U S A. 2020;117:21847-21848.
- Sperber GH. Dental wear: attrition, erosion, and abrasion-a palaeo-odontological approach. Dent J. 2017;5:E19.
- Kruzic JJ, Hoffman M, Arsecularatne JA. Fatigue and wear of human tooth enamel: A review. J Mech Behav Biomed Mater. 2023;138:105574.
- Schlueter N, Amaechi BT, Bartlett D, et al. Terminology of erosive tooth wear: consensus report of a workshop organized by the ORCA and the Cariology Research Group of the IADR. Caries Res. 2020;54:2-6.
- Nascimento MM, Dilbone DA, Pereira PN, Duarte WR, Geraldeli S, Delgado AJ. Abfraction lesions: etiology, diagnosis, and treatment options. Clin Cosmet Investig Dent. 2016;8:79-87.
- Shellis RP, Addy M. The interactions between attrition, abrasion and erosion in tooth wear. Monogr Oral Sci. 2014;25:32-45.
- Li Y, Yu F, Niu L, et al. Associations among bruxism, gastroesophageal reflux disease, and tooth wear. J Clin Med. 2018;7:E417.
- Carvalho TS, Colon P, Ganss C, et al. Consensus report of the European Federation of Conservative Dentistry: erosive tooth wea — diagnosis and management. Clin Oral Investig. 2015;19:1557-1561.
- Bartlett D, O’Toole S. Tooth wear and aging. Aust Dent J. 2019;64 Suppl 1:S59-S62.
- Goldstein G, Goodacre C, MacGregor K. Occlusal vertical dimension: best evidence consensus statement. J Prosthodont Off J Am Coll Prosthodont. 2021;30:12-19.
- Bartlett D, O’Toole S. Tooth wear: best evidence consensus statement. J Prosthodont. 2020 Dec 17.
- Schlueter N, Luka B. Erosive tooth wear – a review on global prevalence and on its prevalence in risk groups. Br Dent J. 2018;224:364-370.
- Clarrett DM, Hachem C. Gastroesophageal reflux disease (GERD). Mo Med. 2018;115:214-218.
- Bohon C. Binge Eating disorder in children and adolescents. Child Adolesc Psychiatr Clin N Am. 2019;28:549-555.
- Udo T, Grilo CM. Prevalence and correlates of DSM-5-defined eating disorders in a nationally representative sample of U.S. adults. Biol Psychiatry. 2018;84:345-354.
- Saads Carvalho T, Lussi A. Chapter 9: Acidic Beverages and Foods Associated with Dental Erosion and Erosive Tooth Wear. Monogr Oral Sci. 2020;28:91-98. doi:10.1159/000455376
- van Rijkom HM, Truin GJ, Frencken JEFM, et al. Prevalence, distribution and background variables of smooth-bordered tooth wear in teenagers in the Hague, the Netherlands. Caries Res. 2002;36:147-154.
- Wiegand A, Attin T. Occupational dental erosion from exposure to acids: a review. Occup Med Oxf Engl. 2007;57:169-176.
- Goldfarb MB, Maupomé G, Hirsh AT, Carvalho JC, Eckert GJ, Hara AT. Dentists clinical decision-making for erosive tooth wear: An online pilot study. J Dent. 2020;100:103424.
- Bartlett D. A personal perspective and update on erosive tooth wear – 10 years on: Part 1 – diagnosis and prevention. Br Dent J. 2016;221:115-119.
- Olley RC, Wilson R, Bartlett D, Moazzez R. Validation of the basic erosive wear examination. Caries Res. 2014;48:51-56.
- Bartlett D, Ganss C, Lussi A. basic erosive wear examination (BEWE): a new scoring system for scientific and clinical needs. Clin Oral Investig. 2008;12 Suppl 1:S65-68.
- Eccles JD. Dental erosion of nonindustrial origin. A clinical survey and classification. J Prosthet Dent. 1979;42:649-653.
- Fares J, Shirodaria S, Chiu K, Ahmad N, Sherriff M, Bartlett D. A new index of tooth wear. Reproducibility and application to a sample of 18- to 30-year-old university students. Caries Res. 2009;43:119-125.
- Margaritis V, Mamai-Homata E, Koletsi-Kounari H, Polychronopoulou A. Evaluation of three different scoring systems for dental erosion: a comparative study in adolescents. J Dent. 2011;39:88-93.
- Lussi A. Dental erosion clinical diagnosis and case history taking. Eur J Oral Sci. 1996;104:191-198.
- Smith BG, Knight JK. An index for measuring the wear of teeth. Br Dent J. 1984;156:435-438.
- Millward A, Shaw L, Smith AJ, Rippin JW, Harrington E. The distribution and severity of tooth wear and the relationship between erosion and dietary constituents in a group of children. Int J Paediatr Dent. 1994;4:151-157.
- Bardsley PF, Taylor S, Milosevic A. Epidemiological studies of tooth wear and dental erosion in 14-year-old children in North West England. Part 1: The relationship with water fluoridation and social deprivation. Br Dent J. 2004;197:413-416.
- Mulic A, Tveit AB, Wang NJ, Hove LH, Espelid I, Skaare AB. Reliability of two clinical scoring systems for dental erosive wear. Caries Res. 2010;44:294-299.
- Eversole SL, Saunders-Burkhardt K, Faller RV. Erosion prevention potential of an over-the-counter stabilized SNF2 dentifrice compared to 5000 ppm F prescription-strength products. J Clin Dent. 2015;26:44-49.
From Dimensions of Dental Hygiene. April/May 2024; 22(3):32-35