
The Role of Nutrition in Dental Caries
Explore the impact of diet on demineralization, oral microbiome, and pH levels in the oral cavity.
This course was published in the April/May 2024 issue and expires May 2027. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
AGD Subject Code: 010
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the relationship between dietary factors and their impact on oral pH levels and demineralization.
- Identify the role of oral bacteria in the development of dental caries and how their activity is influenced by dietary choices.
- List practical approaches for assessing patients’ dietary habits, providing nutritional counseling, and using tools to promote oral health and prevent dental caries.
Oral bacteria play a critical role in the dental caries process. The familiar offenders are Streptococcus mutans, S. sobrinus, and lactobacillus.2 S. mutans will produce lactic acid to lower the pH levels in the oral cavity and glucans to assist in bacteria colonization on enamel. S. sobrinus also contributes to the production of acid and may speed up the demineralization process.3 Lactobacillus will generate acid, further lowering the oral pH levels.4
At a resting (unstimulated) state, oral pH levels are around 7.0. After an eating event involving carbohydrates, the pH of the oral cavity decreases to a significantly low pH level of 5.5. At a pH level of 5.5, demineralization begins and minerals, especially calcium, begin leaching from the enamel. The production of acid is ongoing until the carbohydrate is cleared from the oral cavity.1
Stephan Curve
In the 1940s, Robert M. Stephan, DDS, MS, discovered a link between nutrition and dental caries. His theory was that oral bacteria reacted quickly with carbohydrates to decrease the oral cavity’s pH. He concluded that enamel demineralization occurs below the critical threshold of 5.5 pH following the consumption of fermentable carbohydrates, acidic liquids, or sugar alongside acidogenic bacteria.5 His results demonstrated the oral pH drops to critical acidic levels within the first 3 minutes after consuming a glucose solution. This is known as the “Stephan curve.”
It takes the oral cavity about 20 minutes to buffer the acids produced by bacteria and return to normal pH levels. This means the acidic pH may remain around 5.5 for approximately 20 minutes before it gradually goes back to a normal or resting level. This cyclic process occurs frequently throughout the day as individuals consume various foods and beverages that decrease and increase the oral cavity’s pH.1
The higher the frequency of fermentable carbohydrate consumption, the more acid is produced. When more acid is produced, the oral cavity remains at the critical low pH level longer, thus increasing caries risk. Once carbohydrates are cleared from the oral cavity, the pH level of the oral cavity begins to return to normal — between 6.8 and 7.0. The goal in preventing dental caries and demineralization is to keep pH levels around neutral for the greatest amount of time in a day. Factors that increase the pH levels to neutralize the oral cavity include long lengths of time between eating events involving carbohydrates, sufficient fluoride availability, adequate saliva flow, proper biofilm control, and consumption of cariostatic foods.1
Among two people consuming the same amount of fermentable carbohydrates, the individual who eats or drinks more fermentable carbohydrates frequently throughout the day has the highest caries risk. Using the Stephan curve, if an individual consumed a cookie within 4 minutes, the dentition would be exposed to a significantly low oral pH level of 5.5 for around 40 minutes, until the oral pH level returns to the individual’s normal pH level.6 If another individual eats the same cookie but in four bites with 60 minutes in between each bite, the individual’s dentition would be exposed to a significantly low pH level for around 160 minutes (four eating events x 40 minutes) until the pH level returns to normal.6 Consuming fermentable carbohydrates more frequently throughout the day makes it more difficult for the oral pH level to reach a normal level.
An emerging hypothesis of alkali formation as a factor in oral pH balance has been suggested. Several studies have examined the role of the alkali produced by oral bacteria in caries management.7–9 The arginine deiminase system (ADS) is one pathway for oral bacteria to produce alkali. The ADS pathway and arginine (amino acid) both create ammonia that raises oral pH levels. Arginine and ADS promote less cariogenic oral bacteria and disrupt the biofilm matrix of S. mutans.10 One study reported notably raised levels of arginine deiminase activity in the saliva of individuals without caries activity compared to those individuals with active caries activity.9
Food Properties and Caries Risk
All foods and beverages that contain sugars have the potential to cause caries. Depending on the physical properties, some food items could fall into multiple categories. Nutritional epidemiological studies found that Americans, ages 2 and older, continue to exceed the recommended consumption of added sugars on a daily basis.11 The time needed to clear cariogenic foods from the oral cavity ranges from 20 to 60 minutes and acid production from bacteria continues for 1 to 2 hours.12
Cariogenic foods include foods and beverages with different types of sugars. Bacteria use some sugars, such as fermentable carbohydrates, to produce acid and lower oral pH levels.1,6 These sugars include monosaccharides (eg, glucose and fructose), disaccharides (eg, lactose and sucrose), and human-produced fruit juices, honey, and high-fructose corn syrup. Out of the sugars, sucrose is the highest contributor to caries risk. Sucrose, or table sugar, is one of the most common sources of added sugars.13 Sucrose is used to assemble glucans that promote adherence of bacteria, such as S. mutans, to dental biofilm.6
Liquid forms of cariogenic carbohydrates include sugar-sweetened beverages, sodas, fruit drinks, energy drinks, and sports drinks. These types clear the fastest from the oral cavity — in about 20 to 30 minutes.1
Solid forms of cariogenic carbohydrates are sticky or crunchy in texture, such as chips, crackers, and granola bars. These are retained longer than liquid forms, which means extended exposure to acid in the oral cavity. The approximate time needed to clear these solid forms is 45 to 60 minutes.1
Solid forms may be further classified as starches. Starches are retained longer in the oral cavity due to the need for catabolic reactions of polysaccharide chains. Starchy cariogenic foods include baked goods such as cookies or cakes, white noodles, and breads. They stick into the pits and fissures of dentition and are the most difficult to clear from the oral cavity. Oral clearance time for starchy cariogenic foods is around 60 minutes.1 Raw sugars, such as honey, molasses, and brown sugar, have a similar cariogenicity to sucrose.
Ultraprocessed foods are also cariogenic. This class contains fermentable carbohydrates that are lower in nutrients and fiber and higher in salts, fats, and sugars than natural or processed foods.14 The ingredients in these foods contain many preservatives and additives to lengthen their shelf life. In the American diet, 90% of total added sugars are labeled as ultraprocessed.1 This cariogenic class includes frozen meals, fast foods, instant noodles and soups, packaged potatoes, and sodas.
Some foods may reduce caries potential by decreasing acid production. Licorice, cocoa factor, cranberries, tea, coffee, wine, and probiotics have components that may benefit caries management.1,4,15 Glycyrrhiza, an herb found in licorice, may interfere with S. mutans in the oral cavity.15 Plant polyphenols inhibit acid production and can be found in wine, coffee, tea, and cranberries.4,16
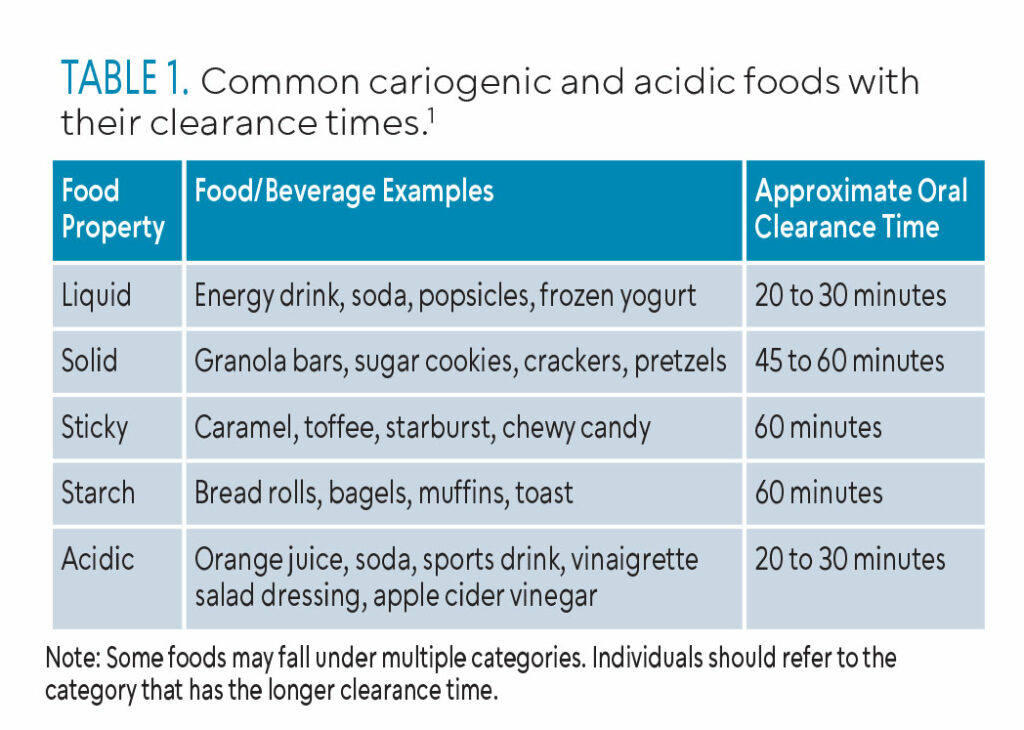 Acidic foods and beverages contain acidic ingredients or have acid by-products from metabolized carbohydrates that contribute to demineralization of enamel or cementum.1,17 These foods have either low pH values themselves or contribute to low pH levels; examples include citric, phosphoric, lactic, ascorbic, and carbonic acids.4 Highly acidic foods include citric fruits (eg, lemons, limes, oranges, yogurts) and noncitric fruits and vegetables (eg, grapes, bananas, cucumbers, green peppers, and sweet potatoes). Highly acidic beverages include nondiet or zero-sugar soft drinks, regular soft drinks, bottled ice teas, sports drinks, energy drinks, and fruit juices. Table 1 lists common cariogenic and acidic foods and beverages with their corresponding clearance times.1
Acidic foods and beverages contain acidic ingredients or have acid by-products from metabolized carbohydrates that contribute to demineralization of enamel or cementum.1,17 These foods have either low pH values themselves or contribute to low pH levels; examples include citric, phosphoric, lactic, ascorbic, and carbonic acids.4 Highly acidic foods include citric fruits (eg, lemons, limes, oranges, yogurts) and noncitric fruits and vegetables (eg, grapes, bananas, cucumbers, green peppers, and sweet potatoes). Highly acidic beverages include nondiet or zero-sugar soft drinks, regular soft drinks, bottled ice teas, sports drinks, energy drinks, and fruit juices. Table 1 lists common cariogenic and acidic foods and beverages with their corresponding clearance times.1
Noncariogenic or cariostatic foods and beverages do not impact the caries process.1 They do not lower the oral pH level below the critical level of 5.5. Foods in this class may have anticariogenic properties that protect against demineralization by either providing fluoride, adding a protective coating on dentition, or aiding as a buffer to an acidic salivary pH level.1
Nonnutritive sweeteners (eg, aspartame, acesulfame, and sucralose) are not metabolized by oral bacteria and do not contribute to an acidic environment. Macronutrients, such as proteins and fats, generally buffer the pH levels. They are ideal to consume when eating or drinking fermentable carbohydrates to balance the oral pH levels.1 Animal proteins, cheeses, nuts, and cow’s milk are examples of cariostatic foods and beverages.18
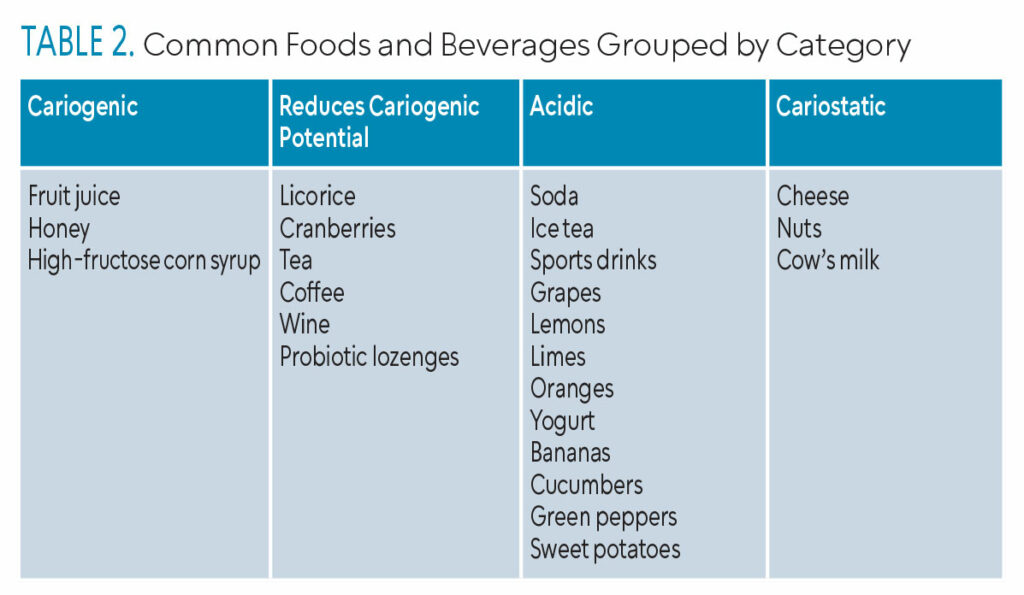 Table 2 groups commonly consumed foods by category.
Table 2 groups commonly consumed foods by category.
The Dental Hygienists’ Role in Nutritional Counseling
Dental hygienists play an important role in helping patients reduce their caries risk. Identifying patients’ risk level is the first step and can be completed via caries risk assessment, which includes a diet questionnaire, or caries management by risk assessment.19
Saliva testing on patients of any age with moderate to high caries risk is appropriate.20 This procedure should take no more than 15 minutes and can be implemented at an initial hygiene appointment or recare appointment.20 The Current Dental Terminology code D0418 may be used for saliva analysis.21 Saliva testing can be done chairside or at home. Findings from saliva testing (resting and stimulated) can reveal pH levels, buffering capacity, saliva volume, and bacteria present in the oral microbiome.22-24
Saliva testing results can help dental hygienists advise patients with acidic levels, low volumes of saliva, and high levels of cariogenic bacteria on what foods and beverages may be contributing to their caries risk. This is the critical time in the appointment when nutritional counseling can be done to help patients reduce exposure to food types that have longer oral clearance times, as well as cariogenic and acidic foods. Furthermore, repeating saliva testing throughout patients’ lifetimes can be beneficial, especially as their caries risk changes.
Patients should be encouraged to reduce their consumption of fermentable carbohydrate-rich foods and beverages, as well as those containing naturally occurring sugars such as honey and fruit juices. The rate at which carbohydrates are cleared from the mouth by saliva plays a crucial role in demineralization prevention; thus, both the consistency and manner of consumption warrant careful consideration. Prolonged exposure to low oral pH levels caused by frequent sipping of sugary drinks or consumption of sticky, sugary foods heightens the risk of demineralization.25
Clinicians may also assess dietary habits by inquiring about recent and typical daily food intake or requesting patients to maintain a food diary over several days. Individuals with a high or frequent consumption of carbohydrates may benefit from additional dietary guidance.26,27
Dietary counseling entails educating patients about the link between cariogenic foods and oral health, as well as the association between consumption frequency and caries susceptibility. Developing a balanced and nutritious eating plan is also crucial. Many online options offer personalized food group recommendations and calorie guidance tailored to an individual’s characteristics such as height, weight, age, sex, and daily activity level.27,28
Conclusion
Dental hygienists as preventive oral healthcare providers are integral in helping patients identify the relationship between nutrition and dental caries. They can complete several assessments relating to caries risk, diet, and salivary diagnostics in order to personalize a caries-prevention approach. These assessments enable clinicians and patients to better understand how patients’ diet and salivary properties affect their caries risk. Nutritional counseling is an extremely important part of caries prevention, especially for high-risk patients.
References
- Sroda R, Reinhard T. Nutrition for Dental Health. 3rd ed. Burlington, Massachusetts: Jones and Bartlett Learning; 2018:438.
- Abranches J, Zeng L, Kajfasz J, et al. Biology of oral streptococci. Microbiol Spectr. 2018;6:10.
- Damle S. Competence and transformation of oral Streptococcus sobrinus in dental caries. Contemp Clin Dent. 2018;9(Suppl 2):S195–196.
- van Loveren C, Broukal Z, Oganessian E. Functional foods/ingredients and dental caries. Eur J Nutr. 2012;51(Suppl 2):S15–25.
- Stephan R, Miller B. A quantitative method for evaluating physical and chemical agents which modify production of acids in bacterial plaques on human teeth. J Dental Res. 1943;22:45–51.
- Stegeman C, Davis J. The Dental Hygienist’s Guide to Nutritional Care. 5th ed. St. Louis: Elsevier;2019:435.
- Kleinberg I. A mixed-bacteria ecological approach to understanding the role of the oral bacteria in dental caries causation: an alternative to Streptococcus mutans and the specific-plaque hypothesis. Crit Rev Oral Biol Med. 2002;13:108–125.
- Liu YL, Nascimento M, Burne R. Progress toward understanding the contribution of alkali generation in dental biofilms to inhibition of dental caries. Int J Oral Sci. 2012;4:135–140.
- Gordan V, Garvan C, Ottenga M, et al. Could alkali production be considered an approach for caries control? Caries Res. 2011;44:547–554.
- He J, Hwang G, Liu Y, et al. l-arginine modifies the exopolysaccharide matrix and thwarts Streptococcus mutans outgrowth within mixed-species oral biofilms. J Bacteriol. 2016;198:2651–2661.
- United States Centers for Disease Control and Prevention. Be Sugar Smart: Limiting Added Sugars Can Improve Health. Available at: .cdc.gov/nutrition/data-statistics/be-sugar-smart.html. Accessed March 25, 2024.
- Linke H, Birkenfeld L. Clearance and metabolism of starch foods in the oral cavity. Ann Nutr Metab. 1999;43:131–139.
- National Library of Medicine. Sucrose. Available at: pubchem.ncbi.nlm.nih.gov/compound/Sucrose. Accessed March 25, 2024.
- Howland J. What is ultra-processed food? Available at: newsnetwork.mayoclinic.org/discussion/mayo-clinic-minute-what-are-ultraprocessed-foods-2/. Accessed March 25, 2024.
- Almaz ME, Sönmez IŞ, Ökte Z, Oba AA. Efficacy of a sugar-free herbal lollipop for reducing salivary Streptococcus mutans levels: a randomized controlled trial. Clin Oral Investig. 2017;21:839–845.
- Ferrazzano GF, Amato I, Ingenito A, Zarrelli A, Pinto G, Pollio A. Plant polyphenols and their anti-cariogenic properties: a review. Molecules. 2011;16:1486–1507.
- Barbour M, Lussi A. Erosion in relation to nutrition and the environment. Monogr Oral Sci. 2014;25:143–54.
- Lussi A, Schlueter N, Rakhmatullina E, Ganss C. Dental erosion — an overview with emphasis on chemical and histopathological aspects. Caries Res. 2011;1:2–12.
- Bowen D, Pieren J. Darby and Walsh: Dental Hygiene Theory and Practice. 5th ed. St. Louis: Elsevier; 2020:1053.
- Dawes C, Wong D. Role of saliva and salivary diagnostics in the advancement of oral health. J Dent Res. 2019;98:133–141.
- American Dental Association. CDT 2023 Coding Companion: Training Guide for the Dental Team. Chicago: American Dental Association; 2021:432.
- Chifor I, Rusu (Dascalu) L, Picos A, et al. Chair-side saliva parameters assessment and caries experience evaluation. Med Pharm Rep. 2019;92(Suppl No 3):S33–38.
- Guo L, Shi W. Salivary biomarkers for caries risk assessment. J Calif Dent Assoc. 2013;41:107-118.
- Rahiotis C, Mitropoulous P, Kakaboura A. Comparative evaluation of chair-side saliva tests according to current dental status in adult patient. Dent J (Basel). 2021;9:10.
- Díaz-Garrido N, Lozano C, Giacaman RA. Frequency of sucrose exposure on the cariogenicity of a biofilm-caries model. Eur J Dent. 2016;10:345–350.
- Marshall T. Impact of diet and nutrition on oral health. Dimensions of Dental Hygiene. 2016;14(4):48–51.
- Hunt AW, Tolle SL. The keys to caries management. Dimensions of Dental Hygiene. 2023;21(2):23.
- United States Department of Agriculture. MyPlate Plan. Available at: myplate.gov/myplate-plan. Accessed March 25, 2024.
From Dimensions of Dental Hygiene. April/May 2024; 22(3):36-41



