The Rise of E-Cigarettes
As the popularity of electronic nicotine-delivery systems grows, dental hygienists need to be prepared to effectively advise patients about the risks of their use.
This course was published in the May 2014 issue and expires May 31, 2017. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Describe the current trends in tobacco use in the United States.
- Define the term electronic cigarette.
- Discuss the regulation of electronic nicotine-delivery systems (ENDS).
- Explain the systemic and oral effects of ENDS.
- Identify evidence-based practice for tobacco cessation.
January 2014 marked the 50th anniversary of the first report of the United States Surgeon General’s Advisory Committee on Smoking and Health, which introduced new knowledge about the effects of smoking on oral and systemic health, disease, morbidity, and mortality.1 Even though much effort has been made to educate the public about the negative health effects of smoking, tobacco use is still the single largest preventable cause of death and disease in the US.
Today, approximately 22% of American adults are cigarette smokers.1 Smoking is most prevalent among men age 25 to 44, nonHispanic American Indians/Alaska natives, and adults living below the federal poverty level.2 Efforts to reduce tobacco use have achieved some success. The overall prevalence of smoking has declined since 2005, and tobacco control programs have reduced smoking in the US.2 Although gains have been made in the fight against tobacco, new challenges, such as the increasing popularity of electronic cigarettes, also called e-cigarettes, have surfaced. In order to best serve their patients and to support efforts to reduce tobacco use, oral health professionals need to remain up-to-date on the use of new tobacco- and nicotine-delivery systems.
ELECTRONIC CIGARETTES DEFINED
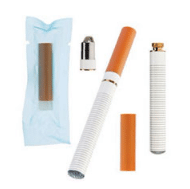
E-cigarettes are electronic nicotine-delivery systems (ENDS) that consist of a cartridge containing nicotine and propylene glycol, an atomizer, and a battery (Figure 1).3 When a user inhales, a pressure-sensitive circuit is activated, which heats the atomizer and vaporizes the liquid as it is brought through the mouthpiece. The vapor consists of a fine mist that does not contain smoke or carbon monoxide and disperses more quickly than traditional cigarette smoke.4 The act of using ENDS is often called “vaping” and users are termed “vapers.”5
The nicotine cartridges used in ENDS come in a variety of flavors, including vanilla, cherry, java, piña colada, and menthol.6 They are also offered in a myriad of nicotine strengths. When e-cigarettes are inhaled, light-emitting diodes are illuminated. Originally, these lights were red, but now they are often blue or another color to differentiate them from traditional cigarettes.7
ENDS were invented by a Chinese pharmacist, Hon Lik, in 2003.5 American users previously could only order ENDS online, but they are now widely available in gas stations, grocery stores, tobacco shops, and shopping malls. Traditional cigarettes are purchased in packs that cost approximately $5.51.8 ENDS are initially purchased as a kit that includes a battery, charger, power cord, and cartridges, which together cost between $59.95 and $129.95. Refill cartridges are then purchased as needed—ranging from $0.50 to $3 each. Disposable ENDS are also available at about $10 each. For a traditional one-pack-per-day smoker, the estimated annual cost is $1,800 vs $874 per year for an ENDS starter kit and refill cartridges. According to ENDS manufacturers, one ENDS refill cartridge is equivalent to one pack of cigarettes; therefore, the cost of using ENDS in comparison to traditional cigarettes is about half (Figure 2).
USERS OF ELECTRONIC NICOTINE-DELIVERY SYSTEMS

About 40% of Americans are aware of ENDS, and it is estimated that 5 million smokers and 1 million former smokers have used these devices. Their use is more popular among young people than older adults.9 Most individuals use ENDS to quit smoking, avoid relapsing after quitting, or to reduce their tobacco use.7 College students, however, do not use ENDS with the intention of quitting conventional cigarettes and actually prefer ENDS use over traditional smoking. They are more likely to try ENDS first, before using traditional cigarettes or other forms of tobacco.10
According to a 2013 survey released by the US Centers for Disease Control and Prevention, ENDS use among teenagers is increasing.11 From 2011 to 2012, the number of high school students who had reported trying ENDS rose from 4.7% to 10%. The number of high school students currently using ENDS increased from 1.5% to 2.8%, and the number of high school students currently using both ENDS and traditional cigarettes increased from 1.2% to 2.2%. In 2013, 7.2% of high school students who had tried ENDS reported never smoking traditional cigarettes, while 80.5% of current ENDS users reported currently using traditional cigarettes, as well.11,12
The act of inhaling and exhaling the vapor is the most pleasurable part of the experience for most ENDS users. ENDS also do not cause breath malodor or leave a lingering smell on hair and clothing, as cigarette smoking does.13 Among adults, the use of ENDS has increased since 2010, with one in five current adult smokers having tried e-cigarettes.14
THE DEBATE OVER GOVERNMENT REGULATION
ENDS are not currently regulated by the US Food and Drug Administration (FDA), which regulates cigarettes and tobacco. In 2008, the FDA attempted to regulate ENDS by blocking their import into the US. The importer challenged the FDA in court in 2009.15 After an appeal in the Federal Court of Appeals in Washington, DC, it was determined that the FDA could not regulate ENDS because they were not marketed as tobacco cessation devices.16 The Family Smoking Prevention and Tobacco Control Act, however, allows state and local governments to regulate the sale or use of tobacco products, including ENDS.16
Currently, the US Department of Transportation prohibits the use of ENDS during air travel.17 Other public places, such as hospitals and universities, have included ENDS in their tobacco-free policies, as well. Although ENDS do not contain tobacco, many public health policies include those products that intend to mimic tobacco delivery systems, contain tobacco flavoring, or provide nicotine for reasons other than tobacco cessation efforts in their definition of “tobacco products.”18–20
Public health agencies strongly discourage the advertising or targeting of ENDS to young people for fear they will serve as a gateway to smoking. The FDA has similar concerns, including the risk for increasing nicotine addiction and the addition of new avenues for children to try tobacco. In 2012, the US Surgeon General reported that each day in the US, 3,800 people younger than 18 smoke their first cigarette and more than 1,000 teenagers go on to become daily cigarette smokers.21 These concerns are also related to children’s ease of access to purchase ENDS. Some states have not implemented legal age restrictions for purchasing ENDS. With enticing tobacco flavors, ENDS could help build another generation of tobacco users and nicotine addicts. Additionally, ENDS are not currently mandated to include health warnings, as is required for traditional cigarettes.6
Advertising of ENDS also remains unregulated in the US. Similar to marketing materials used for traditional cigarettes, advertisements for ENDS often incorporate attractive models and celebrities to suggest that the use of ENDS is glamorous and modern. This type of marketing has proven effective, as the sales of ENDS at the end of 2013 were projected to reach $1 billion. The New York Times reported that one ENDS company spent $12.4 million on ads that included celebrity endorsements in major media for the first quarter of 2013.22
 Starting in 2016, the United Kingdom will regulate ENDS as medicines under the Medicines and Healthcare Products Regulatory Agency. This type of regulation will prohibit the promotion of these products to children and adolescents younger than 16, with a stipulation barring the use of packaging and flavoring that are attractive to young people. The regulation also ensures that ENDS’ long-term safety is monitored.23 France plans to regulate ENDS as tobacco products, banning indoor use and their sale to minors, as well as enforcing certain advertising bans.24 Four countries—Brazil, Uruguay, Seychelles, and Singapore—have banned the manufacturing, use, and import of ENDS.25
Starting in 2016, the United Kingdom will regulate ENDS as medicines under the Medicines and Healthcare Products Regulatory Agency. This type of regulation will prohibit the promotion of these products to children and adolescents younger than 16, with a stipulation barring the use of packaging and flavoring that are attractive to young people. The regulation also ensures that ENDS’ long-term safety is monitored.23 France plans to regulate ENDS as tobacco products, banning indoor use and their sale to minors, as well as enforcing certain advertising bans.24 Four countries—Brazil, Uruguay, Seychelles, and Singapore—have banned the manufacturing, use, and import of ENDS.25
ENVIRONMENTAL EFFECTS
Little research has been conducted about the effects of ENDS on the environment, and what is available has produced conflicting results. ENDS produces a vapor that contains volatile organic compounds and particles.26 McAuley et al27 compared the pollutants expelled with ENDS to traditional cigarettes. The results showed that ENDS produced minimal amounts of pollutants, compared to cigarette smoking. Another study found that particles from ENDS vapor remain present in room air even after use is discontinued, similar to second-hand smoke.26 It is likely that those within close proximity to ENDS users inhale vapors second hand.
ENDS are not currently characterized as a tobacco cessation device, medicine, or medical device, so there are no regulation requirements for manufacturing at an approved site or with quality control of each device or batch of solution. In the absence of these types of regulations, manufacturers may change product designs and contents as they choose, which creates inconsistent products, and impedes the ability to conduct high-quality research.28
SYSTEMIC HEALTH
Just as the environmental effects of ENDS need to be further researched, the systemic effects of ENDS also require additional study. Systemic effects of traditional cigarettes have been well established and include high exposure to carbon monoxide, increased heart rate, high nicotine serum concentrations, and growth in peripheral airway flow resistance. Goniewicz et al29 evaluated the vapors generated from 12 brands of ENDS in controlled conditions using a smoking machine. This study found that ENDS vapors contained toxic substances, but the levels were nine times to 450 times lower than traditional cigarette smoke.29 Vansickel et al30 found that ENDS exposed users to no measureable levels of nicotine or carbon monoxide and did not increase their heart rates.
The research on how much nicotine is absorbed into the blood stream is conflicting. Serum nicotine levels have been found to be much lower with the use of ENDS vs traditional cigarettes.31 Conversely, another study found that ENDS produce nicotine levels similar to traditional cigarettes.32 Research that examined the vacuum pressures (puffing) required to use ENDS found that they require a higher vacuum pressure than traditional cigarettes.33 Eissenberg34 also evaluated the variation in vacuum pressures needed to smoke several different ENDS devices, and found a decrease in the dosage of nicotine as the ENDS cartridge was used—and that even with an increase in vacuum pressure, the nicotine dosage was highly variable. Erratic dosing combined with the need for stronger inhalation could be contributing factors to low nicotine serum levels after using an ENDS device.34 An in vitro study by Zhang et al35 suggested that some nicotine was deposited into lung alveoli, which was likely absorbed into the arteries of ENDS users. Nicotine is probably present in the lungs at lower levels than occurs with traditional cigarettes due to variations in the particle size and density within the inhaled vapor of ENDS. The vacuum pressure required to smoke ENDS, inconsistent manufacturing and labeling of ENDS cartridges, and variations in vapor particle size and density during inhalation attribute to the widely varied nicotine absorption amounts of ENDS devices. Further research needs to be conducted.35
Although serum nicotine levels for ENDS users appear similar to or less than traditional cigarettes, there is another factor to consider in regard to safety and systemic effects of ENDS. The nicotine content of ENDS cartridges typically ranges from 6 mg to 24 mg; however, they can contain up to 100 mg. Nicotine, in addition to being addictive, can be lethal in excess (?0.5 mg to 1 mg of nicotine/kg of weight). For example, if a child weighing 30 kg swallows the contents of one 24 mg ENDS cartridge, acute nicotine poisoning could result, which can lead to death.36 Conversely, traditional cigarettes contain about 1.5% nicotine by weight, which equates to 1 mg to 2 mg of bioavailable nicotine per cigarette.37–39
Of the minimal research that is available on ENDS, a common conclusion is that the top priority is to identify the safety (systemic, environmental) of short- and long-term ENDS use.28 The evidence on the systemic impact of ENDS is minimal and conflicting, and there is no definitive knowledge on how ENDS affect the body. As such, health care providers should not recommend the use of ENDS as a tobacco cessation tool or intervention due to this lack of available evidence.
ORAL HEALTH
Like the systemic effect of ENDS, there is little evidence on the oral effects of ENDS use. Polosa et al13 examined some of the adverse oral effects of ENDS in a 2011 study. The study revealed that after using ENDS for 4 weeks, 6% of patients reported mouth irritation, 8% noted sore throat and dry mouth, and 9% reported mouth ulcers. After 8 weeks of use, 8% reported a dry cough. After 24 weeks, 8% complained of throat irritation and 7% had dry mouth. Overall, the incidence of adverse oral effects was small, but it appears that ENDS use does exert negative effects on the oral cavity. The effect of ENDS on periodontal diseases and healing has not been researched. More study is needed in order to identify the long-term effects of ENDS on the environment, the body, and the oral cavity.
CLINICAL RELEVANCE
Dental hygienists need to remain up-to-date on ENDS use, particularly regarding its effects on oral and systemic health. Commonly, medical histories ask about tobacco use, but patients might not reveal their ENDS use without prompting from oral health professionals.
Currently, ENDS is not a proven method of tobacco cessation, even though many consumers use them for this reason. Traditional nicotine replacement therapies are separated from the pleasurable forms of tobacco use.5 Clean nicotine (lacking combustion products or other tobacco plant toxins) in the form of nicotine replacement therapies has been used for 30 years and is a safe way to support smoking cessation. These therapies, however, are less satisfying for smokers, who enjoy receiving the nicotine through smoke or vapor, and their efficacy is low.40,41 The possibility of creating a clean nicotine inhalation device has been discussed by researchers for some time as a potentially more effective way to promote smoking cessation. Due to the lack of proven safety or effectiveness, however, ENDS cannot be utilized in this capacity.42 Dental hygienists need to educate their patients on this lack of evidence related to ENDS, while remaining abreast on the positions of public health agencies in regards to ENDS safety.
Dental hygienists should continue to support tobacco cessation through evidence-based methods, such as counseling and medications.43 The American Dental Hygienists’ Association’s (ADHA) “Ask, Advise, Refer” program is a national tobacco intervention initiative designed to promote cessation by dental hygienists.44 Available at askadviserefer.org, this program follows the most successful steps to aid patients in quitting smoking, including: an in-depth presentation on the effects of tobacco and nicotine; step-by-step guide on questioning smokers; tips on advising them of why quitting is recommended; specific referrals to local quit lines; and options for Web-based cessation programs. Also, a variety of in-office handouts and reference sheets is available for immediate download or by request from the ADHA website for clinicians.
The “Ask, Advise, Refer” program recommends offering some type of smoking cessation medication in addition to a behavioral program. One option is a traditional nicotine replacement therapy, or pharmacotherapy. FDA-approved traditional nicotine replacement therapy products include gum, lozenges, transdermal patches, nasal sprays, and oral inhalers. Bupropion SR and varenicline are medications used to aid in cessation.44
CONCLUSION
One of the objectives of Healthy People 2020 is to increase tobacco screening in dental care settings. The target is for 58.2% of general practice dentists to report that they or members of their dental teams ask patients about their tobacco use. Currently 52.9% report doing so.45
As dental hygienists are key to oral health promotion, they should stay current on tobacco and nicotine use trends and evidence-based interventions to aid their patients in eliminating tobacco and nicotine use from their lives.
The use of e-cigarettes is alluring to many people. Many ENDS users believe these tobacco products are cleaner than traditional tobacco. They also don’t cause breath, hair, and clothing to smell bad, and they do not carry the same social stigma as cigarette smoking. For these reasons, the number of ENDS users will likely grow.13
Without definitive information about the safety of ENDS devices, parents and health care providers should address the topic of ENDS with adolescents when discussing the dangers of traditional cigarette smoking.
REFERENCES
- Centers for Disease Control and Prevention. Surgeon General’s Report on Smoking and Health 50th Anniversary Report. Available at: cdc.gov/tobacco/data_statistics/sgr/50th-anniversary/index.htm. Accessed April 22, 2014.
- Agaku I, King B, Dube SR, Office on Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention. Current cigarette smoking among adults—United States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61:889–894.
- Ayers JW, Ribisl KM, Brownstein JS. Tracking the rise in popularity of electronic nicotine delivery systems (electronic cigarettes) using search query surveillance. Am J Prev Med. 2011;40:448–453.
- Cobb NK, Byron MJ, Abrams DB, Shields PG. Novel nicotine delivery systems and public health: the rise of the “e-cigarette.” Am J Public Health. 2010;100: 2340–2342.
- Bell K, Keane H. Nicotine control: e-cigarettes, smoking and addiction. Int J Drug Policy. 2012;23: 242–247.
- Food and Drug Administration. FDA warns of health risks posed by e-cigarettes. Available at: fda.gov/ downloads/forconsumers/consumerupdates/UCM173430.pdf. Accessed April 21, 2014.
- Foulds J, Veldheer S, Berg A. Electronic cigarettes (e-cigs): views of aficionados and clinical/public health perspectives. Int J Clin Pract. 2011;65:1037–1042.
- American Lung Association Smoking Cessation: The Economic Benefits. Available at: lung.org/stop-smoking/tobacco-control-advocacy/reports-resources/cessation-economic-benefits/states/united-states.html. Accessed April 21, 2014.
- Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. E-cigarette awareness, use, and harm perceptions in US adults. Am J Pub Health. 2012;102:1758–1766.
- Sutfin EL, McCoy TP, Morrell HER, Hoeppner BB, Wolfson M. Electronic cigarette use by college students. Drug Alcohol Depend. 2013;131:214–221.
- Corey C, Wang B, Johnson SE, et al. Electronic cigarette use among middle and high school students—United States, 2011-2012. MMWR Morb Mortal Wkly Rep. 2013;62:729–730.
- National Youth Tobacco Survey. Methodology Report. 2011. Available at: cdc.gov/tobacco/ data_statistics/surveys/nyts. Accessed April 21, 2014.
- Polosa R, Caponnetto P, Morjaria JB, Papale G, Campagna D, Russo C. Effect of an electronic nicotine delivery device (e-cigarette) on smoking reduction and cessation: a prospective 6-moth pilot study. BMC Public Health. 2011;11:786.
- King BA, Alam S, Promoff G, Arrazola R, Dube SR. Awareness and ever-use of electronic cigarettes among U.S. adults, 2010-2011. Nicotine Tob Res. 2013;15:1623–1627.
- Sottera Inc vs FDA, No.10-5032 (D.C. Cir 2010). Available at: blogs.findlaw.com/dc_circuit/2010/12/ sottera-inc-v-fda-no-10-5032.html. Accessed April 21, 2014.
- 21 United States Code. Title 21—Food And DrugsChapter 9—Federal Food, Drug, And Cosmetic ActSubchapter Ix—Tobacco Products Sec 387 – Definitions. Available at: gpo.gov/fdsys/granule/USCODE-2010-title21/USCODE-2010-title21-chap9-subchapIX-sec387/content-detail.html. Accessed April 21, 2014.
- United States Department of Transportation. Policy on e-cigarettes. Available at: dot.gov/sites/dot.dev/files/ docs/PolicyOnECigarettes.pdf. Accessed April 21, 2014.
- The Ohio State University Human Resources Department. OSU Tobacco Free Policy. Available at: hr.osu.edu/public/documents/policy/policy720.pdf. Accessed April 21, 2014.
- University of Kentucky Policy. Available at: uky.edu/TobaccoFree. Accessed April 21, 2014.
- Central Michigan University Policy. Available at: cm-life.com/2010/08/19/electronic-cigarettes-banned-in-campus-buildings-residence-halls. Accessed April 21, 2014.
- United States Department of Health and Human Services. Preventing Tobacco Use Among Youth and Young Adults: A Report of the Surgeon General. Available at: surgeongeneral.gov/library/reports/ preventing-youth-tobacco-use/full-report.pdf. Accessed April 21, 2014.
- Elliott S. E-cigarette makers’ ads echo tobacco’s heyday. New York Times. August, 29 2013. Available at: nytimes.com/2013/08/30/business/media/e-cigarette-makers-ads-echo-tobaccos-heyday.html?_r=1&. Accessed April 21, 2014.
- Torjesen I. E-cigarettes are to be regulated as medicines from 2016. BMJ. 2013;346:f3859.
- Cahn Z. France acts on electronic cigarettes. J Pub Health Policy. 2013;4:560–564.
- World Health Organization Framework Convention on Tobacco Control. Electronic nicotine delivery systems, including electronic cigarettes. Conference of the Parties to the WHO Framework Convention on Tobacco Control Fifth session. Available at: apps.who.int/gb/fctc/PDF/cop5/FCTC_COP5_13-en.pdf. Accessed April 21, 2014.
- Schripp T, Markewitz D, Uhde E, Salthammer T. Does e-cigarette consumption cause passive vaping? Indoor Air. 2012;2:25–31.
- McAuley TR, Hopke PK, Zhao J, Babain S. Comparison of the effects of e-cigarette vapor and cigarette smoke on indoor air quality. Inhal Toxicol. 2012;24:850–857.
- Etter JF, Bullen C, Flouris AD, Laugensen M, Eissenberg T. Electronic nicotine delivery systems: a research agenda. Tob Control. 2011;20:243–248.
- Goniewicz M, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapor from electronic cigarettes. Tob Control. 2014;23:133–139.
- Vansickel AR, Cobb CO, Weaver MF, et al. A clinical laboratory model for evaluating the acute effects of electronic “cigarettes”: nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiol Biomarkers Prev. 2010;19:1945–1953.
- Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e-cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomized cross-over trial. Tob Control. 2010;19:98–103.
- Etter JF, Bullen C. Saliva continine levels in users of electronic cigarettes. Eur Respir J. 2011;38:1219–1236.
- Trtchounian A, Williams M, Talbot P. conventional and electronic cigarettes (e-cigarettes) have different smoking characteristics. Nicotine Tob Res. 2010;12:905–912.
- Eissenberg T. Electronic nicotine delivery devices: ineffective nicotine delivery and craving suppression after acute administration. Tob Control. 2010;19:87–88.
- Zhang Y, Sumner W, Chen DR. in vitro particle size distributions in electronic and conventional cigarette aerosols suggest comparable deposition patterns. Nicotine Tob Res. 2013;12:501–508.
- World Health Organization Tobacco Free Initiative. Questions and answers on electronic cigarettes or electronic nicotine delivery systems (ENDS). Available at: who.int/tobacco/communications/ statements/electronic_cigarettes/en. Accessed April 21, 2014.
- Kozlowski L, Mehta N, Sweeney C, et al. Filter ventilation and nicotine content of tobacco in cigarettes from Canada, the United Kingdom, and the United States. Tob Control. 1998;7:369–375.
- Benowitz NL, Jacob P 3rd. Daily intake of nicotine during cigarette smoking. Clin Pharmacol Ther. 1984; 35:499–504.
- Hukkanen J, Jacob P 3rd, and Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115.
- Caponnetto P, Russo C, Bruno CM, Alamo A, Amaradio MD, Polosa R. Electronic cigarette: a possible substitute for cigarette dependence. Monaldi Arch Chest Dis. 2013;79:12–19.
- Casella G, Caponnetto P, Polosa R. Therapeutic advances in the treatment of nicotine addiction: present and future. Ther Adv Chronic Dis. 2010;1:95–106.
- Benowitz NL, Goniewicz ML. The regulatory challenge of electronic cigarettes. JAMA. 2013;310:685–686.
- Foulds J, Schmelzer AC, Steinberg MB. Treating tobacco dependence as a chronic illness and a key modifiable predictor of disease. Int J Clin Pract. 2010;64:142–146.
- American Dental Hygienists’ Association. Ask Advise Refer. Available at: askadviserefer.org. Accessed April 21, 2014.
- Healthy People 2020. Objectives. Available at: healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=41. Accessed April 21, 2014.
From Dimensions of Dental Hygiene. May 2014;12(5):46–50.

