 JOYCE GRACE/ISTOCK/GETTY IMAGES PLUS
JOYCE GRACE/ISTOCK/GETTY IMAGES PLUS
What You Need to Know About Dermal Fillers
While the utilization of these cosmetic enhancements has become more prevalent in the dental setting, their use is not without risk.
This course was published in the November 2021 issue and expires November 2024. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Identify the types of dermal fillers available and their indications for use.
- List the most common complications following injection of dermal fillers.
- Discuss the prevention and management of the most common complication of dermal fillers.
Injectable dermal fillers are widely used materials in nonsurgical cosmetic dermatology and plastic surgery. A report by the American Society of Plastic Surgeons projects an annual increase of 3% in sales of these soft tissue fillers for use in several million patients.1 Among cosmetic procedures, dermal fillers rank second in popularity behind botulinum-toxin injections.2 Along with a continued uptick in dermal filler use, the type of medical provider that can provide such injections has also broadened and currently includes dental professionals. Like any medical procedure, however, there are inherent risks involved.
Dermal fillers are injected into the mid and deep dermal layers to correct moderate to severe wrinkles and folds such as the nasolabial folds, marionette grooves, and deep scars.3 Current fillers can rebalance facial proportions, increase symmetry, and reduce wrinkles and volume loss.4 In dentistry, dermal fillers are used to improve peri-oral soft tissues as an alternative to esthetic crown lengthening, especially to correct cases of a “gummy smile.” Creating an esthetic smile line with fuller lips can help mask excess gingival display. Reconstruction of the lip volume contributes to proper phonetics and retention of removable prostheses.5 Additionally, fillers are implemented to reduce the unesthetic “black triangles” formed between anterior teeth following gingival recession and to enhance the interdental papillae noninvasively.6,7 A few clinical reports have shown promising esthetic results using fillers for reconstruction of interproximal papilla.8–12 They have also been used to manage angular cheilitis by filling the perioral tissues.13
Classification of Dermal Fillers
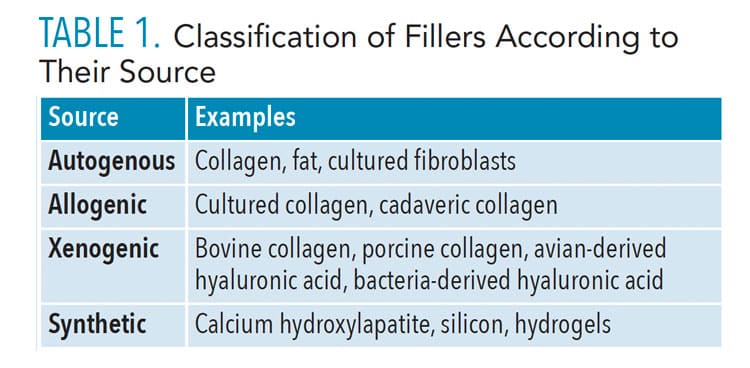
The ideal dermal filler should be safe, yield predictable and lasting results, simple to use, nonmigratory to adjacent structures, and cost effective.14 Currently, no dermal filler meets all these criteria. Current fillers are divided into four major categories based on their source of origin (Table 1). Table 2 lists the United States Food and Drug Administration (FDA)-approved dermal fillers in three categories (page 28).3
Autogenous fat. Adipose tissue has been used as an injectable autogenous graft in corrective and plastic surgery since the late 1800s, typically harvested from the abdomen or buttocks.15 Autogenous fat grafts require two surgical sites: donor and recipient. The procured fatty material is also viscous, requiring a large bore needle for its proper delivery, resulting in bruising, swelling, and tenderness at the recipient sites.16 Fatty filler’s most significant disadvantage is its low survival rate—30%—1-year post-procedure.17 Most of it is resorbed into the body after that time. It is also associated with an increased risk for vascular complications.18 Due to these shortcomings, autogenous fat is rarely used as a filler in the facial region.
Collagen. A group of proteins found exclusively in the skin and connective tissues of animals, collagen is the major structural component of the skin and oral mucosa. Bovine collagen was the first generation of tissue fillers approved by the FDA. Collagen-based filler products are generally a mixture of type I and type III collagen, and are suspended in a phosphate-buffered physiologic saline solution containing 0.3% lidocaine, which provides anesthesia during injection. However, approximately 3% to 5% of patients may experience a delayed-type hypersensitivity reaction following bovine collagen injection.19 To minimize this risk, two skin sensitivity tests are performed 2 weeks apart, with the last one done at least 4 weeks pre-operatively. Other forms of collagen fillers are human bioengineered or porcine collagen. Human collagens are either derived from cadaver skin or from tissue culture of human fibroblasts. These products significantly reduce the risk of allergy compared with bovine collagen. Despite these improvements, collagen fillers have been replaced by new product types.
 Biosynthetic polymers. The second generation of dermal fillers includes a group of biosynthetic polymers (poly-L-lactic acid [PLLA], calcium hydroxyapatite, an polymethylmethacrylate) combined with a variety of injectable carriers such as hydrogels, beads, and liquids.20 They induce the host fibroblasts to produce native collagen for a long duration post-operatively as compared to allogenic collagen. The most popular products in this group are calcium hydroxyapatite and PLLA. They do not require allergy testing; however, due to their highly viscous nature, intradermal placement can lead to nodule formation (as high as 20% in the lips) and should be avoided.21 As calcium hydroxyapatite and PLLA are slowly resorbed, any placement mistake may have a lasting negative cosmetic outcome. Additionally, with the many advantages of hyaluronic acid (HA), synthetic fillers are no longer widely used.
Biosynthetic polymers. The second generation of dermal fillers includes a group of biosynthetic polymers (poly-L-lactic acid [PLLA], calcium hydroxyapatite, an polymethylmethacrylate) combined with a variety of injectable carriers such as hydrogels, beads, and liquids.20 They induce the host fibroblasts to produce native collagen for a long duration post-operatively as compared to allogenic collagen. The most popular products in this group are calcium hydroxyapatite and PLLA. They do not require allergy testing; however, due to their highly viscous nature, intradermal placement can lead to nodule formation (as high as 20% in the lips) and should be avoided.21 As calcium hydroxyapatite and PLLA are slowly resorbed, any placement mistake may have a lasting negative cosmetic outcome. Additionally, with the many advantages of hyaluronic acid (HA), synthetic fillers are no longer widely used.
Hyaluronic acid. Encompassing the third generation of dermal fillers, HA products are currently used in 78.3% of all injectable fillers with an expected annual increase of 7.5%, according to the American Society of Plastic Surgeons.1 HA is the main polysaccharide in the extracellular matrix of the human connective tissue naturally found in skin and other tissues. It serves both physical and chemical functions.22 Additionally, since it has affinity to water, HA volumizes, softens, and hydrates the skin. The effects of HA products last up to 6 months to 18 months, depending on the extent of the crosslinking, concentration of the HA, and particle size of various products.23
Adverse Reactions and Complications
The use of dermal fillers is not without risk. Although the incidence of complications is relatively low and most of them are mild, the increase in the number of cosmetic procedures performed has resulted in a concurrent growth in the number of complications.24 For clarity, these complications are classified according to their clinical manifestations: mild, moderate, or severe; pathologic process involved (ischemic vs nonischemic); and time of onset (early or late). Early onset complications typically appear hours to several days post-operatively, while delayed onset complications usually develop weeks to years post injection.
Injection site complications. The most common side effects associated with filler injections, injection site complications can manifest as edema, erythema, itching, ecchymosis, pain, and Tyndall effect. Typically mild, these adverse effects usually last less than a week.25 Pain is another adverse effect. However, the use of a small needle gauge, along with topical anesthetic agents and application of ice prior and after injections can greatly reduce it. Discontinuing aspirin and other nonsteroidal anti-inflammatory drugs along with dietary supplements containing ginkgo biloba, vitamin E, omega-3, fish oil, ginseng, and St. John’s wort at least 1 week prior to the procedure is strongly recommended after consultation with the patient’s primary care provider.26 Additionally, the use of arnica, topical vitamin K, or bromelin may decrease post-injection ecchymosis if used prior to and post-injection.
The Tyndall effect manifests as a small but deep bruise; however, it does not recede over time and requires removal. The Tyndall effect may be caused by placing the HA filler too superficially, which will manifest as bluish discoloration. It can be treated by injecting 15 IU to 50 IU of hyaluronidase (a mucolytic enzyme that hydrolyzes both natural and crosslinked HA dermal fillers).27 Subsequent massage is essential to mechanically mix the hyaluronidase with the HA, promoting hydrolysis.28
Infections. Post-operative infections are relatively rare and are caused by microscopic injury to the dermal barrier during injection.29 The breakage of surface integrity provides a pathway for various pathogenic microorganisms to invade. Practicing a strict aseptic technique during the procedure is essential. The patient should not wear any makeup either before or immediately after the procedure. Additionally, proper skin sterilization with 2% to 4% chlorhexidine or 70% isopropyl alcohol solution prior to the injection is required. Injections into inflamed or infected skin, or through previous layers of filler should be avoided.30
Hypersensitivity. Both immediate (type I hypersensitivity) and delayed (type IV hypersensitivity) reactions following injection of dermal fillers have been reported in the literature.31 A type I reaction can occur within minutes or hours following the injections, and is caused by rapid formation of immunoglobulin E-mediated immune response to the dermal filler. It may present as an angioedema or anaphylactic reaction occurring after initial or repeated exposure.32 The angioedema will usually subside within a few days following intake of antihistamines and/or oral steroids. The patient should be closely monitored to rule out possible infection.
Delayed hypersensitivity reactions are characterized by induration, erythema, and edema and are mediated by T-lymphocytes rather than antibodies. They typically occur 48 hours to 72 hours post-operatively but may be seen as late as several weeks post-injection and can persist for several months.33 Delayed hypersensitivity reactions do not respond to antihistamines. The most effective approach is to remove the offending allergen (filler). If HA was used, treatment with hyaluronidase is recommended. For other types of fillers, a steroid regimen has been recommended until the filler is fully resorbed. Cassuto et al34 recommend the use of laser therapy to liquify the filler, or total excision and removal of the offending agent.
Lumps and nodules. Injecting too much filling material results in post-operative formation of lumps and nodules. Early lumps that form within days or weeks tend to be painless and are most likely the result of suboptimal injection techniques such as use of excess filler, superficial placement, or poor choice of product for the indication.35 Lumps occurring in the early stage of healing may respond to frequent massage of the area. A superficial single nodule due to excessive placement of filler is usually easy to treat with a simple incision and drainage. However, for multiple nodules where surgical removal may not be feasible, enzymatic degradation by hyaluronidase is recommended.28
Dysesthesias, paresthesia, and anesthesia. Inadvertent nerve damage is a rare complication of dermal filler injection, and may be the result of direct nerve injury caused by the needle or the filling material. Some cases of nerve injury may be transient and reversible, while others are permanent. The most common site of nerve injury is the infraorbital foramen. Less frequently, a transient Bell’s palsy of the facial nerve or mandibular nerve dysfunction may last for several weeks.36 However, while 71% of patients with Bell’s palsy experience complete resolution, the remaining 29% exhibit lifelong residual hemifacial weakness. High doses of oral steroids, along with protective strategies of the ocular surface, are recommended for addressing the acute phase of Bell’s palsy.37 Other options include surgical decompression of the meatal segment, antiviral therapy, electrotherapy, physical therapy, and acupuncture. Thorough knowledge of the facial anatomy and innervation by the treating provider are essential to prevent and minimize the incidence of such complications.
 Vascular occlusion. While extremely rare, vascular occlusion requires prompt intervention.38 It has two clinical manifestations: a localized effect resulting in skin necrosis and a distant vascular occlusion causing loss of vision and cerebral ischemic events.39 Arterial occlusion due to intra-arterial injection usually presents with an immediate skin blanching accompanied by pain. If not treated swiftly, the affected skin will develop reticulated erythema, purpura, and ulceration, leading to scarring. Venous occlusion has a more delayed presentation with persistent, dull aching pain, swelling, and a reticulated erythema of the skin.40
Vascular occlusion. While extremely rare, vascular occlusion requires prompt intervention.38 It has two clinical manifestations: a localized effect resulting in skin necrosis and a distant vascular occlusion causing loss of vision and cerebral ischemic events.39 Arterial occlusion due to intra-arterial injection usually presents with an immediate skin blanching accompanied by pain. If not treated swiftly, the affected skin will develop reticulated erythema, purpura, and ulceration, leading to scarring. Venous occlusion has a more delayed presentation with persistent, dull aching pain, swelling, and a reticulated erythema of the skin.40
The glabella is the most common site of necrosis. Small vessels branching from the supratrochlear and supraorbital arteries provide the blood supply to the glabellar region, and the collateral circulation is limited.41 Other high-risk areas include the nasolabial fold, the infraorbital foramen, and zygomaticofacial foramen.38 If signs of tissue necrosis appear, the injection should be stopped, and an immediate injection of hyaluronidase enzyme is crucial to curtain the necrosis and minimize tissue damage.42,43 Its action is greatly enhanced by topical applications of warm compresses (for 5 minutes to 10 minutes every 1 hour to 2 hours) and vigorous massage. Vasodilatation should be stimulated and the filler material should be disbursed.43 The patient should be monitored on a daily basis. If the necrosis is progressing, the patient should undergo hyperbaric oxygen therapy; other measures include topical oxygen therapy, low molecular weight heparin, systemic steroids, and sildenafil platelet-rich plasma. If these measures fail, the filler should be removed followed by intravenous administration of prostaglandin.
Proper wound care is essential to minimize the risk of scarring. Treatment of the resulting scar involves silicone pads and intralesional steroid injection. If a scar remains, it may be treated with light dermabrasion, surgical revision, or injection with filler to restore the contour.38 A distant vascular occlusion can lead to loss of vision or cerebral ischemic events.39 Rare cases of visual impairment and blindness resulting from injection in the glabellar region have been reported.44 They are caused by retrograde flow of intravascular injected material into the ophthalmic artery, obstructing the blood flow of the distal branches that supply the retina and cornea. Patients may present with a sudden blind spot or visual field deficit. In the case of immediate vision loss or ocular pain, the injection should be stopped, and the patient should see an ophthalmologist or an oculoplastic surgeon for urgent retrobulbar injection of hyaluronidase.
Summary
As the use of dermal fillers increases worldwide, the risk for complications is likely to follow. In the US, dental providers in several states are now permitted to administer dermal fillers following completion of approved training courses. This number is expected to increase over the next few years. Patients must be fully aware of the risks of dermal filler injection and provide informed consent to this therapy. A thorough knowledge of anatomy, the properties of the available filling materials, and injection techniques are essential to prevent complications. It is also necessary to detect complications early and manage them effectively to improve patient outcomes.
References
- American Society of Plastic Surgeons. Plastic Surgery Statistics Report. Available at: plastic-surgery.org/news/plastic-surgery-statistics/2014-statistics.html. Accessed October 14, 2021.
- American Society for Aesthetic Plastic Surgery. Cosmetic Surgery National Databank Statistics.Available at: surgery.org/sites/default/files/ASAPS-2016-Stats.pdf. Accessed October 14, 2021.
- Bray D, Hopkins C, Roberts DN. A review of dermal fillers in facial plastic surgery. Curr Opin Otolaryngol Head Neck Surg. 2010;18:295–302.
- de Maio M. The minimal approach: an innovation in facial cosmetic procedures. Aesthetic Plast Surg. 2004;28:295–300.
- Sinha A, Hurakadli M, Yadav P. Botox and derma fillers: the twin-face of cosmetic dentistry. Int J Contemp Dent Med Rev. 2015:131214.
- Tanwar J, Hungund SA. Hyaluronic acid: Hope of light to black triangles. J Int Soc Prev Commun Dent. 2016;6:497–500.
- Spano SJ, Ghilzon R, Lam DK, et al. Subperiosteal papilla augmentation with a non-animal-derived hyaluronic acid overlay technique. Clin Adv Periodontics. 2020;10:4–9.
- Mansouri SS, Ghasemi M, Salmani Z, Shams N. Clinical application of hyaluronic acid gel for reconstruction of interdental papilla at the esthetic zone. Journal of Islamic Dental Association of Iran. 2013;25(3):208–213.
- Becker W, Gabitov I, Stepanov M, Kois J, Smidt AE, Becker B. Minimally invasive treatment for papillae deficiencies in the esthetic zone: a pilot study. Clin Implant Dent Relat Res. 2010;12:1–8.
- Abdelraouf SA, Dahab OA, Elbarbary A, El-Din AM, Mostafa B. Assessment of hyaluronic acid gel injection in the reconstruction of interdental papilla: a randomized clinical trial. Open Access Maced J Med Sci. 2019;7:1834–1840.
- Patil S, Dhalkari C, Indurkar M. Hyaluronic acid: ray of hope for esthetically challenging black triangles: a case series. Contemp Clin Dent. 2020;11:280–284.
- Awartani F, Tatakis D. Interdental papilla loss: treatment by hyaluronic acid gel injection: a case series. Clin Oral Investig. 2015;20:775–1780.
- Lorenzo-Pouso AI, García-García A, Pérez-Sayáns M. Hyaluronic acid dermal fillers in the management of recurrent angular cheilitis: a case report. Gerodontology. 2018;35:151–154.
- Dastoor SF, Misch CE, Wang HL. Dermal fillers for facial soft tissue augmentation. J Oral Implantol. 2007;33191–204.
- Billings E Jr, May JW Jr. Historical review and present status of free fat graft autotransplantation in plastic and reconstructive surgery. Plast Reconstr Surg. 1989;83:368–381.
- Bellini E, Grieco MP, Raposio E. The science behind autologous fat grafting. Ann Med Surg (Lond). 2017;24:65–73.
- Report on autologous fat transplantation. ASPRS Ad-Hoc committee on new procedures. Plast Surg Nurs. 1987;7:140–141.
- Sito G, Manzoni V, Sommariva R. Vascular complications after facial filler injection: a literature review and meta-analysis. J Clin Aesthet Dermatol. 2019;12:E65–E72.
- McCoy JP Jr, Schade WJ, Siegle RJ, et al. Characterization of the humoral immune response to bovine collagen implants. Arch Dermatol. 1985;121:990–994.
- Buck DW 2nd, Alam M, Kim JY. Injectable fillers for facial rejuvenation: a review. J Plast Reconstr Aesthet Surg. 2009;62:11–18.
- Sklar JA, White SM. Radiance FN: a new soft tissue filler. Dermatol Surg. 2004; 30:764–768.
- Garg HG, Hales CA. Chemistry and Biology of Hyaluronan. Amsterdam: Elsevier; 2004.
- Narins RS, Brandt FS, Lorenc ZP, et al. Twelve-month persistency of a novel ribose-cross-linked collagen dermal filler. Dermatol Surg. 2008;34 Suppl 1:S31–39.
- De Boulle K, Heydenrych I. Patient factors influencing dermal filler complications: prevention, assessment, and treatment. Clin Cosmet Investig Dermatol. 2015;8:205–214.
- Lafaille P, Benedetto A. Fillers: contraindications, side effects and precautions. J Cutaneous Aesthet Surg. 2010;3:16–19.
- Gilbert E, Hui A, Meehan S, et al. The basic science of dermal fillers: past and present. Part II: adverse effects. J Drugs Dermatol. 2012;11:1069–1077.
- Douse-Dean T, Jacob CI. Fast and easy treatment for reduction of the Tyndall effect secondary to cosmetic use of hyaluronic acid. J Drugs Dermatol. 2008;7:281–283.
- DeLorenzi C. Complications of injectable fillers, part I. Aesthet Surg J. 2013;33:561‐575.
- Cohen JL. Understanding, avoiding, and managing dermal filler complications. Dermatol Surg. 2008;34(Suppl 1):S92–S99.
- Bailey SH, Cohen JL, Kenkel JM. Etiology, prevention, and treatment of dermal filler complications. Aesthet Surg J. 2011;31:110–121.
- Bellman B. Immediate and delayed hypersensitivity reactions to restylane. Aesthet Surg J. 2005;25:489–491.
- Alijotas-Reig J, Fernández-Figueras MT, Puig L. Late-onset inflammatory adverse reactions related to soft tissue filler injections. Clin Rev Allergy Immunol. 2013;45:97–108.
- Arron ST, Neuhaus IM. Persistent delayed-type hypersensitivity reaction to injectable non-animal-stabilized hyaluronic acid. J Cosmet Dermatol. 2007;6:167–171.
- Cassuto D, Marangoni O, De Santis G, et al. Advanced laser techniques for filler-induced complications. Dermatol Surg. 2009;35:1689–1695.
- Sclafani AP, Fagien S. Treatment of injectable soft tissue filler complications. Dermatol Surg. 2009;35:1672–1680.
- Fitzgerald R, Bertucci V, Sykes JM, et al. Adverse reactions to injectable fillers. Facial Plast Surg. 2016;32:532–555.
- Glass GE, Tzafetta K. Optimising treatment of Bell’s palsy in primary care: the need for early appropriate referral. Br J Gen Pract. 2014;64:807–809.
- Souza Felix Bravo B, Klotz De Almeida Balassiano L, et al. Delayed-type necrosis after soft-tissue augmentation with hyaluronic acid. J Clin Aesthet Dermatol. 2015;8:42–47.
- DeLorenzi C. Complications of injectable fillers, part 2: vascular complications. Aesthet Surg J. 2014;34:584–600.
- Sclafani AP, Fagien S. Treatment of injectable soft tissue filler complications. Dermatol Surg. 2009;35:1672–1680.
- Hirsch RJ, Cohen JL, Carruthers JD. Successful management of an unusual presentation of impending necrosis following a hyaluronic acid injection embolus and a proposed algorithm for management with hyaluronidase. Dermatol Surg. 2007;33:357–360.
- Beer K, Downie J, Beer J. A treatment protocol for vascular occlusion from particulate soft tissue augmentation. J Clin Aesthet Dermatol. 2012;5:44–47.
- Kassir R, Kolluru A, Kassir M. Extensive necrosis after injection of hyaluronic acid filler: case report and review of the literature. J Cosmet Dermatol. 2011;10:224–231.
- Kim D, Eom J, Kim JY. Temporary blindness after an anterior chamber cosmetic filler injection. Aesth Plast Surg. 2015;39:428–430.
From Dimensions of Dental Hygiene. November 2021;19(11)26-28, 31.



