 TASSII/E+/GETTY IMAGES PLUS
TASSII/E+/GETTY IMAGES PLUS
CE Sponsored by Colgate in Partnership with the American Academy of Periodontology—Maintaining the Oral Microbiome
As one of the most colonized areas of the body, clinicians need to understand how best to promote health and prevent dysbiosis in the oral microbiome.
This course was published in the March 2019 issue and expires March 2022. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the development of the oral microbiome and microbiome dysbiosis.
- Identify the characteristics of the oral microbiome in health and disease.
- List strategies for maintaining a healthy oral microbiome.
INTRODUCTION
The evolving understanding of oral biology and the role of a healthy oral microbiome, as well as various factors that may lead to a dysbiotic state, drive the need to consider what will be “the next generation of prevention.” In the oral cavity, billions of microbes comprise an ecosystem that colonizes both the hard tooth surfaces and the soft mucosa tissues. Evidence shows that decreasing bacteria in the whole mouth through the use of antimicrobials correlates with significant reductions in plaque, gingivitis, and other harmful biofilm-related oral diseases. To achieve and maintain oral health, it is necessary to elevate the importance of fighting harmful bacteria on 100% of mouth surfaces, which will lead to better oral health outcomes for all patients. This article reviews the development and characteristics of the oral microbiome and provides a list of strategies for maintaining a healthy oral microbiome. I am sure it will be a valuable resource in pursuing a proactive and preventive approach in your practice.
The Colgate-Palmolive Company is delighted to have provided an unrestricted educational grant to support this article “Maintaining the Oral Microbiome” in this continuing education series created in collaboration with the American Academy of Periodontology.
—Matilde Hernández, DDS, MS, MBA
Scientific Affairs Manager
Colgate Oral Pharmaceuticals
FROM THE AMERICAN ACADEMY OF PERIODONTOLOGY
The oral microbiome is the foundation for health within and beyond the mouth. Imbalances in this environment can have major implications for a person’s periodontal status, cardiovascular wellness, and digestive system. As such, it’s important for patients to understand the overall value of an oral hygiene routine, healthy diet and exercise habits, and care from a periodontist.
In this article, educators and American Academy of Periodontology (AAP) periodontists Kevin Luan, BDS, MS, and Aniruddh Narvekar, BDS, outline just how patient education and professional care can keep the microbiome healthy.
The AAP is proud to work with Dimensions of Dental Hygiene and Colgate-Palmolive to bring you insights that will keep your patients well.
—Richard T. Kao, DDS, PhD
President, American Academy of Periodontology
This two-unit CE course is developed in collaboration with the American Academy of Periodontology and is supported Through An unrestricted educational grant from Colgate.
The intimate relationship between the microbiome and the host is dynamic and influenced by many environmental factors such as diet, tobacco usage, and stress. These— along with many other aspects of modern lifestyle—influence and alter our microbiome and its respective properties that cause a shift within the body’s ecosystem from a balance of health to disease, and vice versa. The oral cavity is one of the most heavily colonized parts of the human body and, therefore, susceptible to this shift equally if not more so than other areas of the body. To counteract this shift for disease prevention, clinicians must focus on the host and its microbiome residents as one, rather than separate entities.
The oral cavity is inhabited by a vast and diverse mixture of microorganisms, including viruses, protozoa, fungi, archaea, and bacteria.1 To curate and record this information, the Forsyth Institute developed the Human Oral Microbiome Database in 2007. This online expanded database of human-associated microbiomes provides the scientific community with tools and information to help understand the role of microbiomes in health and disease. Currently, 770 microbial species have been enumerated, of which 57% are officially named, 13% unnamed but cultivated, and 30% are known as uncultivated phylotypes. The samples collected that make up this database are derived from a mixture of healthy subjects and subjects with more than a dozen disease states ranging from caries, periodontal diseases, endodontic infections, and cancer.
ORAL MICROBIOME DEVELOPMENT
There is evidence that a variance exists in the development of the oral microbiome at birth, depending on whether the child has a vaginal or caesarean-section delivery, resulting in an exposure of different types of microorganisms.2 One study showed that vaginally-born children have a higher number and more diverse microbiome 3 months after birth when compared with Caesarean-section born children.3 Additionally, 3-month-old breastfed infants show evidence of higher colonization with oral lactobacilli than infants fed with formula.4
The mouth hosts a variety of bacteria due to the distinct habitats such as the tongue, attached gingiva, gingival sulcus, teeth, cheeks, lips, and hard and soft palates, which allow for microbial colonization.5 Therefore, the oral cavity represents a heterogenous ecological system that supports different microbiome communities.6 The moist areas of the mouth offer nutrients, such as salivary proteins, gingival crevicular fluid, and glycoproteins, that support the growth of many microorganisms.7 As teeth erupt, microbial colonization occurs almost instantly on new surfaces, which starts a major ecological event in the mouth of a child.8 This occurs again as primary teeth are replaced with their respective succedaneous teeth, which significantly alters the oral microbiome.9 The occlusal surface of teeth, including the pits and fissures, provide a shelter for microorganisms to persist in an extensive biofilm formation.7 Fixed dental prosthesis, including dentures, crown and bridgework, and implant restorations, can also influence the formation of a biofilm as well as affecting the composition of the microbiome.10–12
MICROBIOME DYSBIOSIS
The host-immune response to pathogenic bacterial species has long been understood to result in periodontal diseases.13 The dysbiosis, or microbial imbalance, of pathogenic bacteria results in oral microbiota that cause a negative and destructive relationship in the oral cavity.14,15 Many studies have successfully identified individual and specific oral microbiota that differ between health and disease.16–20 However, it has also been established that after periodontal treatment, the pathogenic species associated with periodontal disease tend to decrease.21 Additionally, there is a general trend for health-associated species to increase after periodontal treatment.22 Recent studies show that the shift of health-compatible to disease-associated microbiome is due to the relative proportional increase in pathogenic bacteria, and not due to the transmission of new bacteria from person-to-person.23 This host-microbe interaction is particularly important in patients with immunosuppression, who are at greater risk of fungal infections and potentially fatal viral infections of the mucous membranes, as well as nonoral species causing oral infections.24–26
HEALTHY ORAL MICROBIOME MAINTENANCE
With the establishment of the oral microbiome, a process of maintaining homeostasis is regulated by resident bacteria through anti-inflammatory and pro-inflammatory responses to its environment, relative to the location within the oral cavity.27 Fortunately, in addition to the action of bacteria, the oral cavity contains saliva and gingival crevicular fluid to provide nutrients for the growth of microbes and aid in antimicrobial activities.28,29 One study identified 108 different microorganisms per milliliter of saliva, especially in areas such as the tongue.30 Saliva helps to promote oral health by facilitating in swallowing, mastication, and aiding digestion using specialized proteins and enzymes to help maintain a healthy oral microbiome.7 For example, saliva’s ability to convert dietary nitrates from oral bacteria to nitric oxide inhibits the growth of specific cariogenic bacteria, helping to protect against the development of dental caries.31 Enzymes act on the tooth surface acquired pellicle to trigger and mediate bacterial adherence.32 In addition to this, saliva helps modulate layers of plaque, which controls biofilm development and activity.33 Plaque biofilm can be removed naturally via the muscles of the tongue and cheeks during mastication and speech, as well as debridement with a toothbrush or a scaler.
THE ORAL MICROBIOME IN HEALTH AND DISEASE
The lack of a healthy oral microbiome can contribute to negative health effects. For example, certain oral bacteria help catalyze dietary nitrates, most commonly from vegetables, fruit, and processed meats, to salivary nitrates. Once ingested, these salivary nitrates are further converted to nitric oxide, a potent vasodilator that plays a crucial role in cardiovascular health.34 According to a study at the Mayo Clinic, modest consumption of nitrates has been shown to reduce blood pressure, reduce endothelial dysfunction, and inhibit platelet function.35 Nitrates also help to stimulate gastric mucous secretions from the stomach wall, which protects it from acid and digestive enzymes within the stomach lumen.36
A disturbance in the homeostasis of the oral microbiome can negatively impact the host, resulting in dysbiosis or a state associated with disease.37 This change can be the result of a physiological change such as age or hormones (eg, puberty or pregnancy).38 Changes in salivary flow and/or composition, poor oral hygiene, gingival inflammation, and smoking are some modifiable factors that can also affect the oral microbiome, causing dysbiosis.39,40 Pathogenic bacteria can be found in healthy sites but in relatively lower numbers compared with nonpathogenic bacteria. However, in dysbiosis, there is a significantly higher proportion of pathogenic bacteria and less nonpathogenic bacteria associated with the site.41 Specific structures—such as pits and fissures or exposed root surfaces—enable microbes to accumulate within a dental plaque biofilm, which would have otherwise been shed naturally or removed with good oral hygiene.42
DENTAL CARIES DEVELOPMENT
In the early 19th century, the nonspecific plaque hypothesis was developed, initially stating that dental infections were caused by nonspecific excess growth of all bacteria in dental plaque.43 This was further developed into the ecological plaque hypothesis, which attempted to explain the relationship of the oral microbiota, oral diseases, and the host environment.44 Changes in the respective local environment can alter the competitiveness of certain bacteria, resulting in the proliferation of bacteria that are readily suited to adapt to a new environment. In the case of caries development, the increase in sugar intake frequency with or without a reduction in salivary flow, results in an acidic and lower pH environment for a longer time.45 As a result of this new environment, organisms that are more tolerant of an acidic environment and those that produce acids themselves will proliferate and increase relative to organisms that cannot survive in acidic environments (Figure 1).
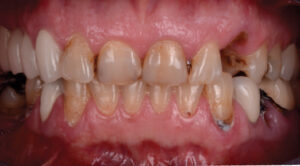
PERIODONTAL DISEASES
The ecological plaque hypothesis can also explain the development of periodontal diseases. The accumulation of pathogenic microbes can cause an inflammatory state by affecting the host immune system, leading to gingivitis and periodontitis.46 However, the presence of biofilm alone is not enough to drive disease progression to periodontitis, as complex interactions between the immune response and biofilm are required in order to shift from a reversible gingivitis to irreversible periodontitis.47 The accumulated biofilm causes a localized inflammatory response that increases the flow of nutrient-rich gingival crevicular fluid and bleeding. This deprives bacteria of oxygen at the inflamed site, allowing for the proliferation of obligatory anaerobic and protein dependent bacteria in the gingival crevice such as Porphyromonas gingivalis and Tannerella forsythia.48 As a result, the host inflammatory immune response induces destructive mechanisms on the periodontal tissues, which, in turn, continues to breakdown nutrients for the pathogenic bacteria, continuing toward more severe periodontal disease.49 Understanding this hypothesis allows ora health professionals to change the ecological environment in order to inhibit pathogenic bacteria, preventing the proliferation of bacteria.
ORAL CANCERS
The development of oral cancer is associated with alterations in genes, systemic health status, environmental factors, etc. However, emerging evidence has suggested that a link exists between oral cancer and the microbiome.50 The direct metabolism by bacteria to generate carcinogens and the inlammatory effects of certain microorganisms can contribute to the development of cancer, as well as other chronic diseases.51 For example, there is an association of Helicobacter pylori with gastric cancer,52 as well as other cancers including colon, gallbladder, prostate, and lung.53,54
REESTABLISH THE HEALTHY MICROBIOME
There is diversity between the microbiomes of healthy individuals.55 An increase in the colonization and development of specific pathogenic bacteria can be related to the relative abundance of bacteria in the local environment—both from the oral cavity and the external environment.56 Therefore, maintaining a clean environment, consuming a nutritious diet, and creating healthy habits can reduce the probability of pathogens disseminating from the environment, and daily oral hygiene and regular professional prophylaxis can effectively control the relative abundance of pathogens in the oral environment. Figure 2 shows how multiple approaches can aid in maintaining a healthy oral microbiome through the following means.
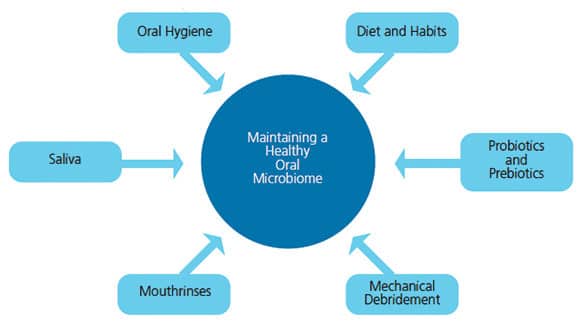
1. Oral hygiene. According to the American Dental Association, general recommendations for all patients include toothbrushing twice daily using either a manual or power toothbrush to remove plaque from all surfaces and the tongue.57 Recent studies show that regular oral hygiene instruction, toothbrushing, interdental cleaning, and rinsing with an antimicrobial mouthrinse, such as one containing 0.075% cetylpyridinium chloride (CPC) have been demonstrated to significantly reduce the amount of pathologic plaque bacteria, eliminate supragingival plaque, and improve gingival health when compared with toothbrushing alone.58
2. Diet and habits. The consumption of refined carbohydrates results in microbial fermentation that produces lactic acid and, thus, a more acidic environment that shifts the oral microbiome toward caries-causing bacteria such as S. mutans, Lachnospiraceae, Veillonellaceae, and Actinomycetales.59 As health care providers, it is our duty to provide diet and nutritional counseling to our patients to promote a more healthy oral environment. A recent study showed drinking alcohol may influence the bacterial composition by causing a depletion of beneficial commensal bacteria and an increased colonization of potentially pathogenic bacteria in the oral cavity.60 Such changes could potentially contribute to several diseases such as periodontitis, head and neck cancer, and digestive tract cancers. A study by Wu et al,61 showed smoking may promote a more anaerobic oral environment and change the oral bacterial community, promoting a more pathogen-friendly environment, increasing the risk for oral diseases.
3. Probiotics and prebiotics. Probiotics contain live microorganisms that are intended to improve the microbiome by replenishing the beneficial microbiota and increasing their biodiversity. Although they were developed to change the gut microbiota, limited evidence has shown they could be used to prevent periodontal diseases. A study on probiotics containing Lactobacillus reuteri showed efficacy in reducing both gingivitis and plaque in patients with moderate to severe gingivitis.62
Prebiotics act as food for the microbiota already present. Probiotics undergo a fermentation process that feeds the beneficial bacterial colonies. This helps to increase the number of desirable bacteria and is associated with better health and reduced disease risk.
4. Mechanical debridement. A prolonged interval between periodontal therapy appointments allows for the pathogenic microbiome to recolonize from the untreated diseased sites to the recently treated diseased sites.63,64 Patients with periodontal diseases should be treated presumably through standard periodontal care (plaque control, scaling and root planing) in a single visit when possible in order to prevent the recolonization of pathogenic bacteria in treated sites.23
5. Mouthrinses. A recent study showed an increase in the pH in the oral cavity following the use of chlorhexidine and povidone iodine rinses. The use of a variety of mouthrinses, including chlrohexidine and povidone iodine, was shown to decrease the number of S. mutans, decrease caries activity, and reduce the plaque index in the oral cavity.65 Another study by Akande et al66 on mouthrinses, showed twice daily use of mouthrinses containing CPC reduces oral microbial load counts in healthy subjects when used as an adjunct to their normal oral hygiene procedures.
6. Saliva. Saliva plays an important role in maintaining a healthy oral environment. Some of saliva’s functions include maintaining a favorable pH for microbial growth, removing exogenous substrates, and providing a continuous supply of endogenous nutrients for the beneficial resident oral microbiota.67
Strategies to increase salivary flow include chewing sugar-free gums and using products containing nonfermentable sweeteners. Sugar alcohols, such as sorbitol, erythritol, and xylitol, have been shown to reduce the incidence of caries.68
CONCLUSION
A healthy oral microbiome can be maintained via patient education and professional intervention. Without the provision of professional oral health care, oral microbiome dysbiosis may result, leading to dental caries and periodontal disease, in addition to affecting general health.69–71
REFERENCES
- Wade WG. The oral microbiome in health and disease. Pharmacol Res. 2013;69:137–143
- Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107:11971–11975.
- Lif HP, Harnevik L, Hernell O, et al. Mode of birth delivery affects oral microbiota in infants. J Dent Res. 2011;90:1183–1188.
- Holgerson PL, Vestman NR, Claesson R, et al. Oral microbial profile discriminates breast-fed from formula-fed infants. J Pediatr Gastroenterol Nutr. 2013;56:127–136.
- Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010;192:5002–5017.
- Xu X, He J, Xue J et al. Oral cavity contains distinct niches with dynamic microbial communities. Environ Microbiol. 2015;17:699–710.
- van’t Hof W, Veerman EC, Nieuw Amerongen AV, Ligtenberg AJ. Antimicrobial defense systems in saliva. Monogr Oral Sci. 2014;24:40–51.
- Sampaio-Maia B, Monteiro-Silva F. Acquisition and maturation of oral microbiome throughout childhood: An update. Dent Res J (Isfahan). 2014;11:291–301.
- Marsh PD, Devine DA. How is the development of dental biofilms influenced by the host? J Clin Periodontol. 2011;38 (Suppl 11):28–35.
- Busscher HJ, Rinastiti M, Siswomihardjo W, van der Mei HC. Biofilm formation on dental restorative and implant materials. J Dent Res. 2010;89:657–665.
- Hannig C, Hannig M. The oral cavity—a key system to understand substratum-dependent bioadhesion on solid surfaces in man. Clin Oral Investig. 2009;13:123–139.
- Øilo M, Bakken V. Biofilm and dental biomaterials. Materials (Basel). 2015;8:2887–2900.
- Hajishengallis G, Liang S, Payne MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10:497–506.
- Hajishengallis G, Darveau RP, Curtis MA. The keystone pathogen hypothesis. Nat Rev Microbiol. 2012;10:717–25.
- Darveau RP, Hajishengallis G, Curtis MA. Porphyromonas gingivalis as a potential community activist for disease. J Dent Res. 2012;91:816–820.
- Abusleme L, Dupuy AK, Dutzan N, et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013;7:1016–1025.
- Griffen AL, Beall CJ, Campbell JH, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012;6:1176–1185.
- Paster BJ, Boches SK, Galvin JL, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–3783.
- Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000. 2006;42:80–87.
- Yang F, Zeng X, Ning K, et al. Saliva microbiomes distinguish caries-active from healthy human populations. ISME J. 2012;6:1–10.
- Laksmana T, Kittichotirat W, Huang Y, Chen W, Jorgensen M, Bumgarner R, et al. Metagenomic analysis of subgingival microbiota following non-surgical periodontal therapy: a pilot study. Open Dent J. 2012;6:255–261.
- Shi B, Chang M, Martin J, et al Dynamic changes in the subgingival microbiome and their potential for diagnosis and prognosis of periodontitis. MBio. 2015;6:e01926–01914.
- Chen C, Hemme C, Beleno J, et al. Oral microbiota of periodontal health and disease and their changes after nonsurgical periodontal therapy. ISME J. 2018;12:1210–1224.
- Petti S, Polimeni A, Berloco PB, Scully C. Orofacial diseases in solid organ and hematopoietic stem cell transplant recipients. Oral Dis. 2013;19:18–36.
- Soga Y, Maeda Y, Ishimaru F et al. Bacterial substitution of coagulase-negative staphylococci for streptococci on the oral mucosa after hematopoietic cell transplantation. Support Care Cancer. 2011;19:995–1000.
- Diaz PI, Hong BY, Frias-Lopez J, et al. Transplantation-associated long-term immunosuppression promotes oral colonization by potentially opportunistic pathogens without impacting other members of the salivary bacteriome. Clin Vaccine Immunol. 2013;20:920–930.
- Devine DA, Marsh PD, Meade J. Modulation of host responses by oral commensal bacteria. J Oral Microbiol. 2015;7:26941.
- Grant MM, Creese AJ, Barr G, et al. Proteomic analysis of a noninvasive human model of acute inflammation and its resolution: the twenty-one day gingivitis model. J Proteome Res. 2010;9:4732–4744.
- Barnes VM, Teles R, Trivedi HM, et al. Acceleration of purine degradation by periodontal diseases. J Dent Res. 2009;88:851–855.
- Marsh PD, Do T, Beighton D, Devine D A. Influence of saliva on the oral microbiota. Periodontol 2000. 2016;70:80–92.
- Doel JJ, Hector MP, Amirtham CV, et al. Protective effect of salivary nitrate and microbial nitrate reductase activity against caries. Eur J Oral Sci. 2004;112:424–428.
- Hannig C, Hannig M, Attin T. Enzymes in the acquired enamel pellicle. Eur J Oral Sci. 2005;113:2–13.
- Amerongen AV, Veerman EC. Saliva—the defender of the oral cavity. Oral Dis. 2002;8:12–22.
- Kapil V, Webb A J, Ahluwalia A. Inorganic nitrate and the cardiovascular system. Heart. 2010;96:1703–1709.
- Lundberg JO, Carlstrom M, Larsen FJ, Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc Res. 2011;89:525–532.
- Lundberg J O, Gladwin M T, Ahluwalia A et al. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5:865–869.
- Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet. 2012;13:260–270.
- Zaura E, ten Cate JM. Towards understanding oral health. Caries Res. 2015;49(Suppl 1):55–61.
- Marsh PD, Head DA, Devine DA. Prospects of oral disease control in the future —an opinion. J Oral Microbiol. 2014;6:26176.
- Wu J, Peters BA, Dominianni C, et al. Cigarette smoking and the oral microbiome in a large study of American adults. ISME J. 2016;10:2435–2446.
- Marsh PD, Head DA, Devine DA. Ecological approaches to oral biofilms: control without killing. Caries Res. 2015;49(Suppl 1):46–54.
- Marcotte H, Lavoie MC. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol Mol Biol Rev. 1998;62:71–109.
- Theilade E. The non-specific theory in microbial etiology of inflammatory periodontal diseases. J Clin Periodontol. 1986;13:905–911.
- Marsh PD. Are dental diseases examples of ecological catastrophes? Microbiology. 2003;149:279–294.
- Liu YL, Nascimento M, Burne RA. Progress toward understanding the contribution of alkali generation in dental biofilms to inhibition of dental caries. Int J Oral Sci. 2012;4:135–140.
- Meyle J, Chapple I. Molecular aspects of the pathogenesis of periodontitis. Periodontol 2000. 2015;69:7–17.
- Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490.
- Marsh PD, Head DA, Devine DA. Ecological approaches to oral biofilms: control without killing. Caries Res. 2015;49(Suppl 1): 46–54.
- Hajishengallis G. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol. 2014;29:248–257.
- Gholizadeh P, Eslami H, Yousefi M, Asgharzadeh M, Aghazadeh M, Kafil HS. Role of oral microbiome on oral cancers, a review. Biomed Pharmacother. 2016;84:552–558.
- Meurman JH. Oral microbiota and cancer. J Oral Microbiol. August 10, 2010;2.
- Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet. 1975;2:58–60.
- Lax AJ, Thomas W. How bacteria could cause cancer: one step at a time. Trends Microbiol. 2002;10:293–299.
- Hooper SJ, Wilson MJ, Crean SJ. Exploring the link between microorganisms and oral cancer: a systematic review of the literature. Head Neck. 2009;31:1228–1239.
- Zhou J, Ning D. Stochastic community assembly: does it matter in microbial ecology? Microbiol Mol Biol Rev. 2017;81:e2–17.
- Hubbell SP. The Unified Neutral Theory Of Biodiversity And Biogeography. Princeton, New Jersey: Princeton University Press; 2001.
- Chapple IL, Van der Weijden F, Doerfer C, et al. Primary prevention of periodontitis: managing gingivitis. J Clin Periodontol. 2015;42 Suppl 16:S71–76.
- Haraszthy VI, Sreenivasan PK. Microbiological and clinical effects of an oral hygiene regimen. Contemp Clin Trials Commun. 2017;8:85–89.
- Costalonga M, Herzberg MC. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol Lett. 2014;162:22–38.
- Fan X, Peters BA, Jacobs EJ, et al. Drinking alcohol is associated with variation in the human oral microbiome in a large study of American adults. Microbiome. 2018;6:59.
- Wu J, Peters BA, Dominianni C, et al. Cigarette smoking and the oral microbiome in a large study of American adults. ISME J. 2016;10:2435–2446.
- Krasse P, Carlsson B, Dahl C, Paulsson A, Nilsson A, Sinkiewicz G. Decreased gum bleeding and reduced gingivitis by the probiotic Lactobacillus reuteri. Swed Dent J. 2006;30:55–60.
- Könönen E, Saarela M, Karjalainen J, Jousimies-Somer H, Alaluusua S, Asikainen S. Transmission of oral Prevotella melaninogenica between a mother and her young child. Oral Microbiol Immunol. 1994;9:310–314.
- Umeda M, Contreras A, Chen C, Bakker I, Slots J. The utility of whole saliva to detect the oral presence of periodontopathic bacteria. J Periodontol. 1998;69:828–833.
- Shin A, Nam S. The effects of various mouthwashes on the oral environment change for oral health care. Available at: alliedacademies.org/ articles/ the-effects-of-various-mouthwashes-on-the-oral-environment-change-for-oral-health-care-10157.html. Accessed February 11, 2019.
- Akande OO, Aderinokun GA, Alada A, Abayomi OI. Efficacy of different brands of mouth rinses on oral bacterial bacterial load count in healthy adults. African Journal of Biomedical Research. 2019;(3):10.
- Marsh PD, Do T, Beighton D, Devine DA. Influence of saliva on the oral microbiota. Periodontol 2000. 2016;70:80–92.
- Mäkinen KK. Sugar alcohols, caries incidence, and remineralization of caries lesions: a literature review. Int J Dent. 2010;2010:981072.
- Han Y W, Wang X. Mobile microbiome: oral bacteria in extra-oral infections and inflammation. J Dent Res. 2013;92:485–491.
- Chapple IL, Genco R. Diabetes and periodontal diseases: consensus report of the Joint EFP/ AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013;40(Suppl 14):S106–S112.
- de Pablo P, Chapple IL, Buckley CD, Dietrich T. Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 2009;5:218–224.
From Dimensions of Dental Hygiene. March 2019;17(3):25–30.



