
Personalize Your Patient Care
A holistic approach to risk assessment can improve outcomes while reducing costs in the treatment of periodontal diseases.
This course was published in the October 2014 issue and October 31, 2017. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Define the personalized medicine approach.
- Describe why the standard interval to care may need to change.
- Identify the role of salivary diagnostics in the personalized medicine paradigm.
The introduction of the Patient Protection and Affordable Care Act in 2010 was designed to create a shift from procedure-based care to a focus on prevention, risk management, and quality outcomes. Doing so, it was theorized, would help control chronic diseases and the spiraling cost of care—particularly for older adults.1 Provision of unnecessary procedures and services, as well as insufficient emphasis on prevention are major contributors to the economic burden of health care.2 At the same time, a renewed interest in oral health, its impact on overall health, and the cost of care for chronic disease led to the incorporation of oral health goals and objectives in national initiatives (eg, the Surgeon General’s Healthy People 2020). The bottom line is that these changes have meant oral health providers and insurers must ramp up their efforts to change the way care is both provided and paid for now and in the future.
Oral health professionals are well versed in risk assessment and prevention. Providers plan treatment, engage in nutritional counseling, and provide self-care recommendations based on patients’ risk assessments. In recent years, the significant impact of systemic conditions, such as diabetes, on periodontal health has been highlighted. Yet the main tool for managing periodontal risk has long been the biannual recare interval. The majority of patients are placed on this traditional, 6-month preventive recare interval, with appointments every 3-months to 4-months for periodontal maintenance patients.
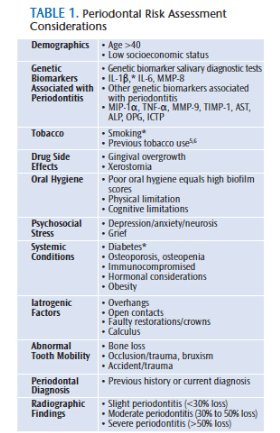
The biannual recare protocol was established in the late 19th century and remains the standard of care today. This remains true, despite the fact that the evidence does not support its use for general application to most patients.3,4 Research does show, however, that regular care is successful in the prevention and management of oral disease.4 Practitioners need to determine the appropriate interval of care based on a holistic approach to periodontal risk assessment.5 In addition to clinical findings, this approach includes the analysis of individual risks,5,6 including genetic predispositions, systemic conditions, and social factors (Table 1). Once patients’ risks are determined, they can be stratified into the appropriate treatment intervals, which is integral to the personalized medicine paradigm.
The personalized medicine approach to treatment eliminates unnecessary recommendations and costs. A major dental insurer recently instituted a pilot program for its employees utilizing this premise. While still entitled to two dental exams annually, employees are now limited to one dental prophylaxis unless the participant has a history of risk, such as pregnancy, diabetes, head and neck radiation, or a previous history of periodontal diseases. Patients who meet these periodontal disease risk criteria will be eligible for up to three additional prophylaxis or maintenance visits annually.7 Assessing individuals’ risk for caries, oral cancer, and periodontal diseases—based on past history and systemic conditions—ensures that patients receive the right care at appropriate intervals. Identifying those at high risk while eliminating unnecessary treatment for people at little-to-no risk stands to decrease cost and improve health outcomes.
Personalized medicine is a shift from traditional practices. While long-established methods have based treatment on outcomes of population studies, personalized medicine approaches patients as individuals with unique risks and biological responses to diseases and treatments.8 The purpose of applying personalized medicine in oral health care is to predict risk and create customized treatment plans—with the end goal being disease prevention and improved outcomes.8 The use of patient stratification based on identified risk factors and biomarkers supports more efficient allocation of resources.9
PATIENT STRATIFICATION FOR PREVENTIVE CARE
In the age of personalized medicine, some familiar preventive care strategies may be subject to change. Results of a study conducted at the University of Michigan suggest that the biannual preventive prophylaxis model may not be applicable to all patients.9
The study looked at the dental insurance claims of 5,117 participants for the presence of any tooth extractions (excluding third molars) over a period of 16 years. Study participants (aged 34 to 55) had no previous history of periodontal diseases and were of average age of disease onset. Participants were categorized by risk factors linked to periodontal diseases—including smoking, diabetes, and interleukin (IL)-1 genetic polymorphisms (primarily IL-1?)—and the number of their respective preventive annual dental visits. Subjects were then stratified by risk into high-risk and low-risk categories. Patients were considered high risk if one or more risk factors were present or deemed low risk if no risk factors were present. Once the patients were stratified by risk, they were further classified by number of preventive visits per year (one or two), which yielded four patient classifications: high risk/one preventive visit, high risk/two preventive visits, low risk/one preventive visit, and low risk/two preventive visits.9
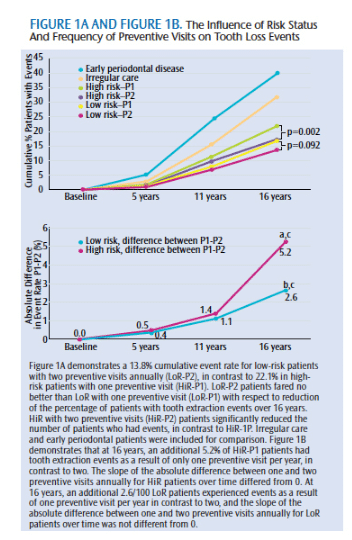
Results of the study demonstrate a significantly higher rate of tooth loss among patients who received preventive care on an irregular basis than those who were regularly seen—indicating that it is critical for patients to have regular preventive dental care (Figure 1A).9 When looking at high-risk participants, the number of risk factors proportionally increased the rate of extraction.9 This revealed that patients with more risk factors appear to be more susceptible to periodontal disease-related tooth loss than those with fewer or no risk factors, thereby suggesting that two preventive visits per year may not be sufficient for those classified as high risk.9
The high-risk/one preventive visit group experienced a significantly higher tooth extraction rate than the high-risk/two preventive visits group, indicating that preventive visits for high-risk patients are beneficial in preventing tooth loss (Figure 1B).9 Although the high-risk/two preventive visits group had fewer teeth extracted than the high-risk/one preventive visit group, this group still had more teeth extracted than the low-risk/two preventive visit group, stressing again that two preventive visits per year may not be sufficient for high-risk patients.9 Participants in the low-risk/one preventive visit group did not have a significantly different rate of tooth extraction than the low-risk/two preventive group, indicating that two preventive visits per year may not be necessary for these patients.
Health care costs continue to rise. Modifying the “one-size-fits-all” model for preventive dental care visits to treating patients based on individual risk has the potential for significant savings. For example, if all 5,117 study participants were seen twice yearly for preventive visits, the total cost over the 16-year study period would have been $16,374,400. If participants observed in this study had been seen based on risk assessment that included a one-time ($150) genetic test, as opposed to the traditional biannual prophylaxis model, a cost savings of more than $2.2 million could have been achieved. If this model were extrapolated to the 175 million insured adults in the United States, the potential savings is almost $5 billion.9
It is important to note that this study focused on stratifications for preventive prophylaxis based on risk factors, but this does not discount the need for an annual oral exam that incorporates screening for oral cancer and caries, in addition to a periodontal evaluation.4 This also includes attention to all aspects of the medical history and their impact on oral health.
IMPLICATIONS FOR PRACTICE
Today, clinicians are encouraged to provide predictive, preventive, personalized, and participatory health care.10 The personalized medicine approach in oral health care has the potential to empower both patients and practitioners by basing the frequency of treatment on the needs of individual patients instead of preset intervals. Conducting risk assessment is a standard of care, as outlined in the American Dental Hygienists’ Association Standards for Clinical Dental Hygiene Practice.11 Applying this theory in a more systematic and personalized approach, however, requires a change in knowledge, skills, and attitudes among oral health providers.12
Salivary diagnostics, when used as a component of risk assessment, identify the biomarkers that support health risk stratification.13 In this new era of personalized medicine, the use of genomics is rapidly moving the discovery of better health outcomes forward. Currently, practitioners use the patient’s health history, family history, and social, environmental, and behavioral risk factors during the assessment phase.12 Based on these data, treatment decisions are made. As thorough as these assessments may be, they only scratch the surface. Not understanding how the individual’s system is handling the microbial challenge or how he or she responds to the recommended treatments in the short- and long-term leaves outcomes to chance.
Genetic dynamics and resulting clinical manifestations to periodontal diseases are based on the host’s response to specific pathogens.13 While there is no individual genetic biomarker for periodontal diseases, research has demonstrated the usefulness in determining pathogen and host-response biomarkers in assessing an individual’s risk for poor periodontal health. The use of salivary diagnostics is often used to pinpoint these biomarkers, such as the IL-1 genetic polymorphisms.14 IL-1, along with other biomarkers, such as tumor necrosis factor (TNF), are host response mediators that are responsible for inflammation and bone resorption.15 Matrix metalloproteinases (MMP) enzymes, which cause connective tissue damage, can also be detected in saliva.15 Additionally, there are several biomarkers associated with specific strains of periodontal bacteria.13 These identifiers can help practitioners determine the best treatment for patients, based on specific bacteria and host-response needs. Therefore, salivary diagnostics as part of a holistic periodontal risk assessment allows for more appropriate stratification of risk.8,9,13
Personalized medicine is most effective when salivary diagnostics is used to determine an individual’s susceptibility to periodontal diseases and how he or she will respond to treatment. This approach to care ensures appropriate treatment is delivered while also reducing disease and cost burden.8,9 The incorporation of such genomic information into periodontal treatment can also increase the level of interprofessional collaboration between oral health providers and health care professionals in the wider health care spectrum.13 Embracing the concept of risk-stratified care management will require a paradigm shift for practitioners, patients, and insuring entities. Identifying and prioritizing patients for targeted interventions in the prevention and management of caries and oral cancer, in addition to periodontal diseases, are important to maximizing health outcomes and the return on investment for those funding care.
Availability and cost of salivary diagnostic tests and the willingness of public and private insurers to recognize these tools as beneficial to overall health will play a pivotal role in the incorporation of genetic testing as a component of risk assessment.16 There are salivary diagnostic tests that identify periodontal pathogens and measure genetic risk for periodontal diseases available today. One test specifically identifies IL-1? and IL-6. As the research in this area continues, focus remains on testing that has adequate specificity and sensitivity. Sensitivity is achieved when a high number of truly positive cases are identified.17 Specificity is achieved when testing identifies a limited number of false-positives.17 For diagnostic usefulness, the ratio for both should be high.17
The optimal use of salivary diagnostics to identify and quantify biomarkers should be considered during an initial risk assessment—prior to biological onset of periodontal disease.14,15,18 These diagnostic tests can also be used to detect the presence of active disease, predict future disease progression, and evaluate the response to periodontal therapy.15 Increasing the number and availability of salivary diagnostic testing mediums that provide genetic information, in addition to placing a greater overall importance on disease risk and prevention, will further support personalized care for patients.
Insurance companies are also looking to shift to a risk-based approach in determining coverage of care.7 As mentioned previously, a major insurance carrier has recently changed their employees’ prophylaxis benefit to once per year.7 Additional visits may be approved if the patient is documented to be at risk for developing periodontal diseases or has been previously diagnosed. Administration of the insurance claim filing and reimbursement related to this new approach is in its infancy. Insurance companies and dental providers are still grappling with the need for a standardized periodontal risk assessment tool, how risk is reported (by provider, patient, or both), and who ultimately makes the determination of the patient’s risk.7
CONCLUSION
In the realm of personalized medicine, oral health professionals are charged with advancing the care they provide. This will require interprofessional skills, clinical experience, and evidence-based knowledge, as well as the ability to speak to patients regarding the effects of risk, including genetics, on their oral health status.13 The incorporation of salivary diagnostics to determine genetic risk and periodontal pathogen susceptibility will empower oral health providers and patients to set individualized preventive care intervals that can result in improved treatment outcomes.
ACKNOWLEDGEMENT
The authors would like to thank Janet Kinney, RDH, MS, and William V. Giannobile, DDS, DMSc, for their support, guidance, and review of this manuscript.
REFERENCES
- Han JE, Aronow HU, Rosario ER, Guenther N. Multidimensional health risk appraisal among adults aging with acquired disabilities. Disabil Health J. 2013;6:195–203.
- Yong PL, Saunders RS, Olsen LA, eds. The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary. Washington, DC: National Academies Press; 2010.
- Teich ST. Risk Assessment-Based Individualized Treatment (RABIT): a comprehensive approach to dental patient recall. J Dent Educ. 2013;77:448–457.
- Giannobile WV, Kornman KS, Williams RC. Personalized medicine enters dentistry: what might this mean for clinical practice? J Am Dent Assoc. 2013;144:874–876.
- Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol 2000. 2013;62:59–94.
- Benton E, Cotter J. The patient who uses tobacco. In: Wilkins EM. Clinical Practice of the Dental Hygienist. 11th ed. Baltimore: Lippincott Williams & Wilkins; 2013:474–495.
- Smiley CJ. Delta Dental’s new plan—bringing risk to plan design. J Mich Dent Assoc. 2014;96:64,66–67.
- Kornman KS, Duff GW. Personalized medicine: will dentistry ride the wave or watch from the beach? J Dent Res. 2012;91(Suppl 7):8S–11S.
- Giannobile WV, Braun TM, Caplis AK, Doucette-Stamm L, Duff GW, Kornman KS. Patient stratification for preventive care in dentistry. J Dent Res. 2013;92:694–701.
- Auffray C, Charron D, Hood L. Predictive, preventive, personalized and participatory medicine: back to the future. Genome Med. 2010;2:57.
- American Dental Hygienists’ Association. Standards for Clinical Dental Hygiene Practice. Chicago: American Dental Hygienists’ Association; 2008.
- Johnson L, Genco RJ, Damsky C, et al. Genetics and its implications for clinical dental practice and education: report of panel 3 of the Macy study. J Dent Educ. 2008;72(Suppl 2):86–94.
- Slavkin HC. Revising the scope of practice for oral health professionals: enter genomics. J Am Dent Assoc. 2014;145:228–230.
- Giannobile WV. Salivary diagnostics for periodontal diseases. J Am Dent Assoc. 2012;143(Suppl 10):6S–11S.
- Taba M Jr, Kinney J, Kim AS, Giannobile WV. Diagnostic biomarkers for oral and periodontal diseases. Dent Clin North Am. 2005;49:551–557.
- Garcia I, Kuska R, Somerman MJ. Expanding the foundation for personalized medicine: implications and challenges for dentistry. J Dent Res. 2013;92(Suppl 7):3S–10S.
- Genco RJ. Salivary diagnostic tests. J Am Dent Assoc. 2012;143(Suppl 10):3S–5S.
- Kinney JS, Morelli T, Braun T, et al. Saliva/pathogen biomarker signatures and periodontal disease progression. J Dent Res. 2011;90:752–758.
From Dimensions of Dental Hygiene. October 2014;12(10):51–54.



