
Reduce the Risk of Implant Failure
The use of locally delivered antimicrobials/antibiotics in conjunction with scaling and root planing may improve the viability of implants at risk of peri-implantitis.
This course was published in the October 2014 issue and October 31, 2017. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Define peri-implantitis.
- Discuss the role of locally delivered antimicrobials/antibiotics in implant therapy.
- Describe the correct protocol for implementing locally delivered antimicrobials/ antibiotics into peri-implantitis treatment.
Implants have become an accepted treatment to replace lost teeth, help increase surface area for chewing, maintain alveolar bone, and restore up to 100% of normal occlusal function. 1 In an ideal world, this occlusal function and the health of the surrounding hard and soft tissue would remain stable. The reality, however, is that some implants fail. As the cost for patients in both time and expense is significant, dental professionals need to be well versed in strategies that support implant health and in recognizing potential problems. Implant failure is caused by either biomechanical or biological factors.2 Biomechanical failures occur due to functional overload of the implant, while biological failures are caused by increases in biofilm accumulation, inflammation, infection, extruded cement, and surgical complications.2 Implant failure is most frequently caused by the biological factor peri-implantitis, which is a devastating inflammatory cycle that causes increased pocket formation, gingival edema and erythema, and destruction of supporting bone.3 Implants are most vulnerable to peri-implantitis and potential failure during the initial 12 months post-placement.2,4 Peri-implantitis affects 5% to 9% of all implants placed.2,4
Proper identification, early diagnosis, and management of peri-implantitis are necessary to furthering implant health. The use of periapical radiographs, accurate probing measurements of periodontal pocket depth, mobility assessment, and clinical evidence of infection are key in the treatment of peri-implantitis. Risk assessment for peri-implantitis is essential to the prevention of dental implant failure. Patients with risk factors—such as diabetes, osteoporosis, past or current smoking, and a history of periodontitis—should be carefully observed throughout the implant treatment and follow-up care process.5,6
ROLE OF LOCALLY DELIVERED ANTIMICROBIALS / ANTIBIOTICS
When efforts to prevent peri-implantitis are not successful, the use of controlled-release local delivery antimicrobials/antibiotics—including chlorhexidine wafers, doxycycline biosorbable gel, and minocycline microspheres—can help reduce biofilm accumulation, possibly decreasing the risk of biological implant failures.2,7 The use of these antimicrobials/antibiotics, in addition to other nonsurgical treatment options, can help reduce the need for surgical intervention.
Scaling and root planing alone may improve inflammation around implants, but research shows this approach only provides minimal improvement in periodontal pocket depth.6 The use of controlled-release drug therapy as an adjunct to nonsurgical periodontal therapy can lead to decreased bleeding on probing, periodontal pocket depth, and inflammation around implants with peri-implantitis.5,7–9 Surgical interventions to reconstruct bone by reducing inflammation with photodynamic therapy (the use of photosensitizing dyes, which are stimulated by light, to eliminate periodontopathogens), curettage to remove granulation tissue, and bone augmentation have integrated locally applied controlled-release antimicrobial/antibiotic therapy to improve outcomes.7,10 Both nonsurgical periodontal therapy and surgical intervention for peri-implantitis rely on the reduction of inflammation due to diminished biofilm, detoxification of the implant surface, and regeneration of attachment for success.10 Systematic reviews, randomized controlled trials, and cohort and case-control studies suggest that the use of local delivery antimicrobials/antibiotics, in addition to nonsurgical periodontal therapy, is more effective at reducing periodontal pocket depth than scaling and root planing alone.7,8,11–17
On the contrary, some studies suggest that the nonsurgical treatment of peri-implantitis is unpredictable, due to the difficulty in fully removing factors contributing to peri-implant inflammation.10 A Cochrane review determined that the evidence is insufficient to support one treatment modality over another as more effective.7 The studies cited in this review, however, did not deny the effectiveness of the treatment modalities reviewed, but rather indicated the need for improved study design and longer follow-up for documentation of patient results.7
Dental hygienists need to approach the treatment of peri-implantitis on a case-by-case basis. The focus of any peri-implantitis treatment plan should be the improvement of surrounding tissue health.
ASSESSMENT
When assessing an implant for peri-implantitis, clinicians must differentiate between peri-implant mucositis and peri-implantitis.18,19 Peri-implant mucositis is the presence of inflammation without discernible bone loss, similar to gingivitis.18,19 Koldsland et al18 defined peri-implant inflammation as a modified sulcus bleeding index score <0 and/or bleeding on probing and bleeding on probing/suppuration with or without the presence of peri-implant bone loss.
Peri-implantitis is the presence of discernible radiographic bone loss and inflammation. The severity and degree of peri-implant bone loss, including its relation to the overall length of the implant and the load-bearing weight the implant will carry, should be considered when diagnosing peri-implantitis. These data are important in assessing the potential for successful treatment intervention with a controlled-release antimicrobial/antibiotic in conjunction with nonsurgical periodontal therapy.18
The possibility of adverse reactions to antimicrobial/antibiotic therapy should be considered during the assessment phase. Those with tetracycline or minocycline allergies, pregnant and nursing women, and patients younger than 15 are contraindicated for the doxycycline biosorbable gel and minocycline microspheres. The use of chlorhexidine wafers should be avoided in patients with sensitivity or allergy to chlorhexidine.
INTERDISCIPLINARY COLLABORATION
Prior to rendering treatment for peri-implantitis with a controlled-release drug therapy, the oral health professional should collaborate with the patient’s dentist and attending specialist (oral surgeon or periodontist). Comprehensive interdisciplinary collaboration is imperative to ensure accuracy of patient assessment, establish history of peri-implant infection/health status, and achieve positive treatment outcomes.20 The dental hygienist should confirm when the dental implant was placed; whether there were any post-surgical complications; receive baseline data to track changes in bleeding on probing, inflammation, and probing measurements; and obtain copies of periapical radiographs and medical history, if available. The oral health professional should review the proposed treatment for peri-implantitis with the patient and obtain an informed consent prior to initiating treatment.
IMPLEMENTATION
After informed consent has been obtained, implementation of controlled-release drug delivery can begin. There are three types of locally delivered antimicrobials/antibiotics to choose from: chlorhexidine wafers, doxycycline biosorbable gel, and minocycline microspheres.
The chlorhexidine wafers contain 2.5 mg of chlorhexidine each and are placed subgingivally with pliers.8 Chlorhexidine is an antiseptic, not an antibiotic, so this approach is well suited for patients with antibiotic allergies. The wafers are indicated for use in pocket depths of 5 mm or greater in conjunction with scaling and root planing. Effective against a wide variety of pathogens, chlorhexidine wafers are effective for 7 days to 10 days and have been shown to successfully reduce pocket depths.14
An additional option is the subgingival application of 10% doxycycline hyclate gel. This product comes with two syringes that are mixed chairside and applied via syringe into the pocket.8 The gel flows freely into the pocket and then hardens once exposed to gingival crevicular fluid. Once applied, the doxycycline is released over 7 days, with effects lasting up to 6 months.8 Studies show that the gel is effective in reducing pocket depth in conjunction with scaling and root planing.15,17
Minocycline microspheres are packaged in a single-dose vial consisting of minocycline hydrochloride (1 mg), intended for use with a syringe provided by the manufacturer. Each single-unit dose is generally enough to fill one to two 4 mm to 6 mm pockets. Exact dosage depends on size, depth, morphology of the implant, and degree of bone loss. The product comes in powder form and should be dispensed from the base of the pocket and continued to extrude until the gingival margin is reached. The microspheres should be used in the treatment of pocket depths ?5 mm. The following case study details the successful treatment of a patient with peri-implantitis using this technique.
Once the controlled-release drug therapy has been administered, the patient is given post-operative instructions specific to the product administered. The recare interval is once per month for 3 consecutive months beginning 30 days post-treatment. At the recare appointment, clinical response to treatment is documented by careful evaluation of bleeding on probing and pocket depth measurements. If clinical evidence of inflammation, bleeding, or a pocket depth of 4 mm or greater exists, continue treatment with the controlled-release drug therapy until the clinical signs of inflammation are resolved or the three consecutive treatment intervals are completed. During the month following the third treatment of the peri-implantitis area, radiographs, pocket depth, and bleeding on probing measurements should be performed.21
Following each treatment appointment, an overview of the care rendered, plans for additional treatment, and outcomes should be reported to the patient’s specialist. By closely managing care and working collaboratively with the specialist, patient compliance is enhanced.20
CASE STUDY
A 58-year-old man presented in an oral surgery practice for a normal post-operative visit to evaluate the status and health of a previously placed implant in area #5. His medical health history was relatively unremarkable. Review of previous chart notes revealed the extraction of a retained root tip #5 with an associated draining fistula. At the initial visit, Class I molar relationship on both the right and left sides was noted, with a Class II tendency on the left side and a deep bite. After the extraction, a 3-month healing time ensued before the implant was placed. Subsequent post-operative examinations revealed integration of the implant with a light contact, no sign of extruded cement, slight inflammation with bleeding on probing, mild gingival edema on the facial aspect of the implant, and periodontal pockets of 2 mm to 4 mm from both the facial and the palatal aspects.
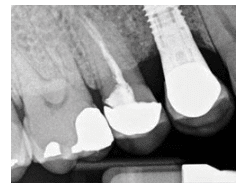
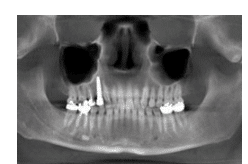
Three years after the final restoration was placed on the implant, the patient noted the presence of bleeding around the implant when using a rubber tip stimulator. Periodontal pockets were present with bleeding on probing (Table 1). The cone-beam computed tomography scan revealed a small radiolucency at the apex of tooth #4 (Figure 1). The periapical radiograph revealed excellent bone-to-implant opposition in area #5, but blunting of the septal bone existed between the fixture area #5 and tooth #4 (Figure 2). Peri-implantitis was diagnosed around implant #5 and periodontal disease around tooth #4.
A treatment plan was proposed and a consent form signed. Both areas were hand scaled with plastic instruments under local anesthesia, and minocycline microspheres were placed subgingivally at both sites. A chlorhexidine mouthrinse was also prescribed.
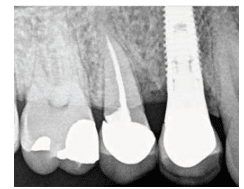
The patient underwent the recommended treatment plan of three subsequent visits with hand instrumentation and subgingivally placed minocycline microspheres. Periodontal probing and bleeding points were taken at each visit with a final post-operative periapical radiograph taken 1 month after treatment was completed. The final evaluation, completed 4 months later, revealed decreased pocket depths and bleeding points on the mesiopalatal of tooth #4 and the distobuccal and distopalatal sites of tooth #5. Radiographically, the implant area #5 appeared stable and well integrated (Figure 3).
At subsequent post-operative follow-ups, the patient continued to present asymptomatically with no clinical signs of inflammation, no bleeding points, and periodontal pockets measuring 2 mm or less with radiographic evidence of crestal bone approximating the first thread on the distal and above the implant table on the mesial of the implant.
CONCLUSION
The treatment of peri-implantitis with controlled-release drug therapy as an adjunct to mechanical instrumentation is an effective modality to decrease infection. Reduction in biofilm accumulation and inflammation around implant areas helps decrease the need for surgical intervention and encourages the potential for implant success.
REFERENCES
- Malo P, de Auaujo Nobre M, Lopes A, Francischone C, Rigolizzo M. All-on-4 immediate-function concept for completely edentulous maxillae: a clinical report on the medium (3 years) and long-term (5 years) outcomes. Clin Implant Dent Relat Res. 2012;14(Suppl 1):139–150.
- Norowski PA Jr, Bumgardner JD. Biomaterial and antibiotic strategies for peri-implantitis: a review. J Biomed Mater Res B Appl Biomater. 2009;88:530–543.
- Avila-Ortiz G. Encouraging success. Dimensions of Dental Hygiene. 2013;11(5):57–62.
- Schmidlin PR, Sahrmann P, Ramel C, et al. Peri-implantitis prevalence and treatment in implant-oriented private practices: a cross-sectional postal and Internet survey. Schweiz Monatsschr Zahnmed. 2012;122:1136–1144.
- Renvert S, Persson GR. Periodontitis as a potential risk factor for peri-implantitis. J Clin Periodontol. 2009;36(Suppl 10):9–14.
- Froum S. Supporting implant success. Dimensions of Dental?Hygiene. 2012;10(2):19–21.
- Esposito M, Grusovin MG, Worthington HV. Interventions for replacing missing teeth: treatment of peri-implantitis. Cochrane Database Syst Rev. 2012;1:CD004970.
- Paquette DW, Ryan ME, Wilder RS. Locally delivered antimicrobials: clinical evidence and relevance. J Dent Hyg. 2008;82(Suppl 3):10–15.
- Renvert S, Samuelsson E, Lindahl C, Persson GR. Mechanical non-surgical treatment of peri-implantitis a double-blind randomized longitudinal clinical study. I: clinical results. J Clin Periodontol. 2009;36:604–609.
- Machtei EE, Frankenthal S, Levi G, et al. Treatment of peri-implantitis using multiple applications of chlorhexidine chips: a double-blind, randomized multi-centre clinical trial. J Clin Periodontol. 2012;39:1198–1205.
- Hanes PJ, Purvis JP, Gunsolley JC. Local anti-infective therapy: pharmacological agents. A systematic review. Ann Periodontol. 2003;8:79–98.
- Gopinath V, Ramakrishnan T, Emmadi P, Ambalavanan N, Mammen B, Vijayalakshmi. Effect of a controlled release device containing minocycline microspheres on the treatment of chronic periodontitis: A comparative study. J Indian Soc Periodontol. 2009;13:79–84.
- Ahamed S, Jalaluddin M, Khalid I, Moon N, Shaf TK, Ali FM. The use of controlled release locally delivered 10% doxycycline hyclate gel as an adjunct to scaling and root planing in the treatment of chronic periodontitis: clinical and microbiological results. J Contemp Dent Pract. 2013;14:1080–1086.
- Jeffcoat MK, Bray KS, Ciancio SG, et al. Adjunctive use of a subgingival controlled–release chlorhexidine chip reduces probing depth and improves attachment level compared with scaling and root planing alone. J Periodontol. 1998;69:989–997.
- Garrett S, Johnson L, Drisko CH, et al. Two multi-center studies evaluating locally delivered doxycycline hyclate, placebo control, oral hygiene, and scaling and root planing in the treatment of periodontitis. J Periodontol. 1999;70:490–503.
- Paquette DW, Oringer R, Lessem J, et al. Locally delivered minocycline microspheres for the treatment of periodontitis in smokers. J Clin Periodontol. 2003;30:787–794.
- Wennstrom JL, Newman HN, MacNeill SR, et al. Utilization of locally delivered doxycycline in non-surgical treatment of chronic periodontitis. A comparative multi-centre trial of 2 treatment approaches. J Clin Periodontol. 2001;28:753–761.
- Koldsland OC, Scheie AA, Aass AM. Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss. J Periodontol. 2010;81:231–238.
- Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008;35(Suppl 8):286–291.
- Vanderbilt AA, Isringhausen KT, Bonwell PB. Interprofessional education: the inclusion of dental hygiene in health care within the United States—a call to action. Adv Med Educ Pract. 2013;4:227–229.
- Ghahroudi RA, Talaeepour A, Mesgarzadeh A, et al. Radiographic vertical bone loss evaluation around dental implants following one year of functional loading. J Dent (Tehran). 2010;7:89–97.
From Dimensions of Dental Hygiene. October 2014;12(10):47–50.



