Maintaining the Balance Between Remineralization and Demineralization
A thorough knowledge of the demineralization/remineralization process is critical to effective caries management.
This course was published in the January/February 2024 issue and expires January/February 2027. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
AGD Subject Code: 010
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Describe community interventions and shifts in caries management that help support remineralization of tooth structure and minimally invasive treatment.
- Explain the processes of demineralization and remineralization, and how scientific understanding of this natural cycle aids efforts to reverse early caries lesions.
- Discuss clinical approaches and materials that lend themselves to minimally invasive care and effective caries prevention and management.
Advances in techniques and materials, while admirable, still required surgical intervention. Dentistry could successfully manage the caries lesion (a symptom of the disease process), but fell short in addressing the disease itself. This tunnel vision often led to a “cycle of rerestoration,” as defined by Brantley et al.1 Caries prevention took a huge step with the introduction of community water fluoridation in 1945, and, in the ensuing years, nearly 74% of the United States population gained access to publicly fluoridated water.2 This exposure greatly aided remineralization — a key process in caries prevention and reversal of early lesions.
Even though fluoride use has expanded greatly, caries continues to be a significant health concern. In fact, restoration of caries ranks among the most common dental procedures. In recent years, a complementary approach to surgical intervention has taken root that focuses on the treatment of caries as a disease process, not just as the end point of a disease. Some have called this the medical model of caries management,3 and among its principles is a thorough evaluation of risk factors and causes.
One of the key tenets relies on healing enamel and dentin at a stage where mechanical repair is not yet necessary; this involves the concept of remineralization and is particularly apt when addressing smooth surface lesions.4 Among the keys to successfully addressing caries is proper patient assessment. One approach has been termed caries management by risk assessment or CAMBRA, as described by Featherstone.5 In this model, the patient’s hygiene, diet, caries activity, and salivary function are assessed and weighed against protective factors, such as fluoride exposure. Caries risk assessment systems such as CAMBRA help support preventive care and minimally invasive treatment, and also facilitate breaking the cycle of continuous restoration.
Caries and Demineralization
Enamel structure is composed of approximately 96% inorganic material, 3% water, and 1% organic material. The inorganic component consists of tightly packed calcium-hydroxyapatite (HA) crystals secreted in a rod-interrod formation. Due to the formation and arrangement of the enamel rod crystals, a porous diffusible space is created between the crystal interfaces. In addition, there is variability within enamel itself, which becomes more porous toward the dentinoenamel junction.
 Caries is the result of a continuous process involving repeated challenges of demineralization and remineralization. A lesion develops when the dynamic changes involved in this cycle shift toward demineralization,4 which is a natural destructive process of hydroxyapatite crystals initiated by a local decrease in pH of the oral cavity. The pH at the outer enamel surface is impacted by the pH of the surrounding environment — saliva, plaque and the acquired enamel pellicle. Organic acids produced by metabolic microbial action on dietary carbohydrates decrease the local pH on the tooth surface and create a concentration gradient for the dissolution of calcium and phosphate ions out of superficial hydroxyapatite crystals. The critical initiation pH for the demineralization of enamel is 5.5 (Figure 1). A decrease in pH in the biofilm and surrounding saliva initiates an acid attack, causing an imbalance in the equilibrium of ions within hydroxyapatite crystals, and their supersaturated content in saliva. Calcium and phosphate ions diffuse from the tooth surface, causing ions to leach into the surrounding biofilm and saliva. This loss is the beginning of the decalcification process, and, if not interrupted, results in the early stages of lesion development.
Caries is the result of a continuous process involving repeated challenges of demineralization and remineralization. A lesion develops when the dynamic changes involved in this cycle shift toward demineralization,4 which is a natural destructive process of hydroxyapatite crystals initiated by a local decrease in pH of the oral cavity. The pH at the outer enamel surface is impacted by the pH of the surrounding environment — saliva, plaque and the acquired enamel pellicle. Organic acids produced by metabolic microbial action on dietary carbohydrates decrease the local pH on the tooth surface and create a concentration gradient for the dissolution of calcium and phosphate ions out of superficial hydroxyapatite crystals. The critical initiation pH for the demineralization of enamel is 5.5 (Figure 1). A decrease in pH in the biofilm and surrounding saliva initiates an acid attack, causing an imbalance in the equilibrium of ions within hydroxyapatite crystals, and their supersaturated content in saliva. Calcium and phosphate ions diffuse from the tooth surface, causing ions to leach into the surrounding biofilm and saliva. This loss is the beginning of the decalcification process, and, if not interrupted, results in the early stages of lesion development.
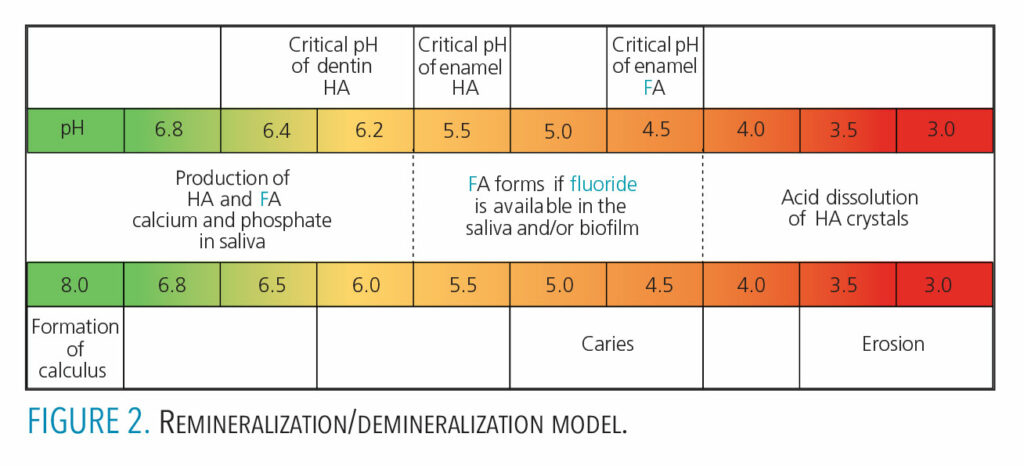 Demineralization begins within the crystalline structure of the enamel and will progress toward the dentin. The lesion advances through the entire enamel layer, damaging the hydroxyapatite crystals in a tapered funnel-like shape. Once demineralization reaches dentin, the process progresses rapidly. The critical pH for the initiation of demineralization in dentin is 6.2 to 6.4, which is higher than that of enamel (Figure 2). Thus, dentin is much more susceptible to demineralization than enamel at similar pH levels. The rate of caries progression depends on a host of factors. Smooth surface caries progresses to cavitation in anywhere from 18 months4 to four years.6
Demineralization begins within the crystalline structure of the enamel and will progress toward the dentin. The lesion advances through the entire enamel layer, damaging the hydroxyapatite crystals in a tapered funnel-like shape. Once demineralization reaches dentin, the process progresses rapidly. The critical pH for the initiation of demineralization in dentin is 6.2 to 6.4, which is higher than that of enamel (Figure 2). Thus, dentin is much more susceptible to demineralization than enamel at similar pH levels. The rate of caries progression depends on a host of factors. Smooth surface caries progresses to cavitation in anywhere from 18 months4 to four years.6
Enamel Remineralization
 Remineralization of enamel occurs naturally in the oral cavity, although the process is much slower than enamel demineralization. This is a natural and continuously occurring phenomenon related to the foods and fluids consumed and the natural mechanism to repair minute damage that might arise from contact with these substances. The remineralization process relies on a neutral pH and is driven by the precipitation of calcium and phosphate ions from saliva, plaque and biofilm back into the enamel. Fluoride facilitates remineralization once the pH exceeds 5.5, assisting in the repair of demineralized enamel or incipient lesions.6 As the pH of the saliva and biofilm becomes more basic, the equilibrium shifts in the opposite direction to precipitate free ions of calcium and phosphate back into the enamel structure (Figure 3).
Remineralization of enamel occurs naturally in the oral cavity, although the process is much slower than enamel demineralization. This is a natural and continuously occurring phenomenon related to the foods and fluids consumed and the natural mechanism to repair minute damage that might arise from contact with these substances. The remineralization process relies on a neutral pH and is driven by the precipitation of calcium and phosphate ions from saliva, plaque and biofilm back into the enamel. Fluoride facilitates remineralization once the pH exceeds 5.5, assisting in the repair of demineralized enamel or incipient lesions.6 As the pH of the saliva and biofilm becomes more basic, the equilibrium shifts in the opposite direction to precipitate free ions of calcium and phosphate back into the enamel structure (Figure 3).
The natural process of remineralization relies on the level of ions present. Various fluoride products or salivary stimulants can be used to speed this process. Fluoride-releasing restorative materials may also be used. In addition, a newer type of material called alkasite was developed as a bulk-fill bioactive restorative capable of releasing fluoride, calcium, and hydroxyl ions to support remineralization. It comes in a powder-liquid formulation and is utilized like other bulk-fill materials, restoring to a depth of 4 mm. The release of the hydroxyl ions helps regulate pH, making it more alkaline, and aids in prevention of demineralization, as well as facilitating the incorporation of the ions into tooth structure.7 There is limited evidence that alkasites have superior esthetics when compared to glass ionomers.
 Using any of these materials requires some type of preparation or surgical intervention on the most serious aspect of the lesion. These materials all contain and release fluoride, which contributes to their effectiveness in remineralizing incipient lesions. As seen in Figure 4, fluoride released from these materials is critical to the process because it is incorporated into hydroxyapatite crystals as fluorapatite. The resulting crystals have a greater resistance to caries development because the critical pH for caries development has now decreased to 4.5, as compared to 5.5 for hydroxyapatite crystals (Figure 2, page 43).6 Other remineralizing agents, such as synthetic nanohydroxyapatite, have also shown success when used in toothpaste. Some chewing gums and mouthrinses may also contain a fluoride booster called casein phosphopeptide-amorphous calcium phosphate (CPP-ACP). This compound helps to keep calcium and phosphate in an amorphous, noncrystalline state. This amorphous state allows for a supersaturation of the minerals to aid in enamel remineralization.8
Using any of these materials requires some type of preparation or surgical intervention on the most serious aspect of the lesion. These materials all contain and release fluoride, which contributes to their effectiveness in remineralizing incipient lesions. As seen in Figure 4, fluoride released from these materials is critical to the process because it is incorporated into hydroxyapatite crystals as fluorapatite. The resulting crystals have a greater resistance to caries development because the critical pH for caries development has now decreased to 4.5, as compared to 5.5 for hydroxyapatite crystals (Figure 2, page 43).6 Other remineralizing agents, such as synthetic nanohydroxyapatite, have also shown success when used in toothpaste. Some chewing gums and mouthrinses may also contain a fluoride booster called casein phosphopeptide-amorphous calcium phosphate (CPP-ACP). This compound helps to keep calcium and phosphate in an amorphous, noncrystalline state. This amorphous state allows for a supersaturation of the minerals to aid in enamel remineralization.8
Gao et al’s8 systematic review of the literature concludes that sodium fluoride has a proven track record in remineralizing enamel caries, and silver diamine fluoride (SDF) has been shown to effectively arrest dentinal caries. Remineralization of dentin caries presents an additional challenge due to the greater organic content and presence of collagen. In vitro studies have demonstrated that SDF has the ability to enhance remineralization of dentin caries due to its ability to prevent the degradation of collagen.9
Saliva is one of the major factors in neutralizing the effects of an acid attack. Saliva has the ability to dilute, wash surfaces, and act as a buffering agent. It also has antibacterial properties and serves as a repository for the ions aiding remineralization.4 When conditions are right, meaning a near neutral pH, saliva can release ions such as calcium, phosphate, and fluoride to facilitate enamel remineralization. Figure 2 (page 43) summarizes the effect pH has on the remineralization/demineralization cycle, and how it affects the dissolution or integration of calcium, phosphate and fluoride into or out of tooth structure.
In addition to releasing ions, saliva also contains valuable proteins, such as statherin, acid proline-rich proteins, and histatins. Statherin is the only salivary protein with the capability to inhibit both primary calcium phosphate precipitation (spontaneous precipitation) and secondary calcium phosphate precipitation (crystal growth).10 This means statherin can help tip the equilibrium toward remineralization by maintaining calcium and phosphate in a supersaturated state, as well as protecting teeth from calcium phosphate growing in unnecessary places.
Future Trends
One promising area of remineralization research involves the use of proteins to not just repair enamel, but also regenerate lost tooth structure. These materials work through the process termed biomimetic remineralization, and include dentin phosphoprotein peptides, self-assembling P11-4 peptides, amelogenin, and poly(amidoamine) dendrimers.11 They are included in toothpastes, gels, and rinses. These agents demonstrate some ability to regenerate enamel in vitro, but more research is needed to prove their clinical efficacy. Synthetic nanohydroxyapatite (nHa) is a biocompatible and bioactive material that has been used since the 1980s in toothpastes and rinses. Experiments using this compound show comparable and sometimes superior results to fluoride therapy as far as remineralization. Some researchers propose that nHa promotes remineralization through deposition of new enamel, while others suggest remineralization is due to the presence of a supersaturated solution of calcium and phosphate. More clinical evidence is needed to prove the superior efficacy of nHa to fluoride.
An additional area of research focuses on fluoride boosters. These solutions are based on varying compositions of calcium and phosphate and are included in toothpastes and chewing gum. This class of products includes CPP-ACP, functionalized beta-tricalcium phosphate, calcium sodium phosphosilicate, and ACP. Some have been used for years, but evidence of clinical effectiveness varies widely.12 Evidence that supports other calcium phosphate solutions is also either weak or demonstrates limited clinical applicability.11 Clearly, further research is indicated.
Sodium trimetaphosphate (STMP) is an additional phosphate solution under investigation. This solution has been added to dentifrices to partly replace fluoride. Recent research demonstrates that a dentifrice and varnish containing STMP and fluoride were superior to a standard fluoride dentifrice and varnish in reducing caries activity.13,14 Again, more research is needed to demonstrate efficacy in the remineralization of early lesions.11
Summary
The gold standard for effective remineralization continues to be agents designed to increase the availability of calcium, phosphate and fluoride at the right time and at the right pH. Products containing these compounds have been proven to work clinically, but, like so much in dentistry, they are dependent on several factors to work effectively — most importantly, patient cooperation. The science of dentistry is clear: remineralization works. Regenerative therapy may be the Holy Grail of preventive dentistry, however, there is much work to be done to bring useful and proven products to market.
The Global Burden of Disease Study (1990–2010) reported that “oral diseases affect 3.9 billion people worldwide and untreated tooth decay affects almost half of the world’s population (44%), making it the most prevalent of the 291 conditions included in this study.”15 Many of these lesions are beyond the scope of remineralization as described in this paper. However, this does not limit the effectives of remineralization therapy. There is a subset of this population that presents with smooth surface lesions at various stages of development. Practitioners must identify patients who can benefit from this therapy, and initiate treatment as early as possible.
When used properly in the younger patient, this can lead to a lifetime of improved oral health and help break the cycle of rerestoration. Dental professionals have always promoted the value of prevention. And while great strides have been made in caries restoration techniques, researchers need to accelerate efforts to understand the mechanisms of the disease process and search for novel techniques and materials that enhance remineralization — and, if possible, regeneration.
Clinicians must also utilize the materials currently available to treat the disease and not just its symptoms. That said, the use of any clinical approach must be based in a fundamental understanding of the cause of the underlying condition — and the science that dictates how the disease process might best be alleviated.
References
- Brantley CF, Bader JD, Shugars DA, Nesbit SP. Does the cycle of restoration lead to larger restorations? J Am Dent Assoc. 1995;126:1407–1413.
- U.S Centers for Disease Control and Prevention. Over 75 Years of Community Water Fluoridation. Available at: https://www.cdc.gov/fluoridation/basics/anniversary.htm. Accessed August 11, 2022.
- Yon MJ, Gao SS, Chen KJ, Duangthip D, Lo ECM, Chu CH. Medical model in caries management. Dent J (Basel). 2019;7:37.
- Ritter AV, Boushell LW, Walter R. Sturdevant’s Art and Science of Operative Dentistry. 7th ed. St. Louis: Elsevier; 2019:40, 61, 84.
- Featherstone JDB. The science and practice of caries prevention. J Am Dent Assoc. 2000;131:887–899.
- Hilton TJ, Ferracane JL, Broome JC. Summitt’s Fundamentals of Operative Dentistry: A Contemporary Approach. 4th ed. Hanover Park, Ill: Quintessence Publishing Co; 2013:97, 98, 390.
- Valencia JJC, Felix VMC, Afrashtehfar KI. Alkasites, a new alternative to amalgam. Report of a clinical case. Acta Scientific Dent Sci. 2019;3:11–19.
- Gao SS, Zhang S, Mei ML, Lo EC, Chu CH. Caries remineralization and arresting effect in children by professionally applied fluoride treatment — a systematic review. BMC Oral Health. 2016;16:12.
- Mei ML, Ito L, Cao Y, Li QL, Lo ECM, Chu CH. Inhibitory effect of silver diamine fluoride on dentine demineralisation and collagen degradation. J Dent. 2013;41:809–817.
- Oppenheim FG, Salih E, Siqueira WL, Zhang W, Helmerhorst EJ. Salivary proteome and its genetic polymorphisms. Ann N Y Acad Sci. 2007;1098:22–50.
- Philip N. State of the art enamel remineralization systems: The next frontier in caries management. Caries Res. 2019;53:284–295.
- Li J, Xie X, Wang Y, et al. Long-term remineralizing effect of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) on early caries lesions in vivo: a systematic review. J Dent. 2014;42:769–777.
- Danelon M, Pessan JP, Nunes Souza Neto F, Rodrigues de Camargo E, Delbem ACB. Effect of toothpaste with nano-sized trimetaphosphate on dental caries: In situ study. J Dent. 2015;43:806–813.
- Manarelli MM, Delbem ACB, Binhardi TDR, Pessan JP. In situ remineralizing effect of fluoride varnishes containing sodium trimetaphosphate. Clin Oral Invest. 2015;19:2141–2146.
- Gonzales TS. Communicable and noncommunicable diseases — a global dialogue. ACD News. 2021;50(2):3.
From Dimensions of Dental Hygiene. Jan/Feb 2024; 22(1):42-45



