 DARDESPOT/E+/GETTY IMAGES PLUS
DARDESPOT/E+/GETTY IMAGES PLUS
Improve Patient Outcomes With Fluoride Therapies
A discussion of the characteristics, benefits, and limitations of fluoride-based therapies in the treatment of caries and dentinal hypersensitivity.
This course was published in the December 2021 issue and expires December 2024. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Explain risk factors for dental caries, as well as fluoride therapies used to prevent or treat caries and dentinal hypersensitivity.
- Discuss the efficacy of fluoride varnish and silver diamine fluoride in caries management.
- Describe the emerging role of ammonium hexafluorosilicate and nano silver fluoride for treating sensitivity, supporting remineralization, and preventing or arresting caries lesions.
Dental caries is the most common and preventable worldwide health issue that can negatively affect quality of life.1 Fluoride-based therapies are often used to prevent or arrest lesions. Currently, 5% sodium fluoride (NaF) and 12% or 38% silver diamine fluoride (SDF) are employed in caries management; however, new fluoride-based therapies—including ammonium hexafluorosilicate (AHF) and nano silver fluoride (NSF)—are emerging as alternative treatment approaches. This article will review the characteristics, benefits, and limitations of various fluoride-based therapies designed to improve patient outcomes.
Caries is a complex, chronic disease that results from a combination of genetic and environmental factors.2 Streptococcus mutans are among the chief microbes in caries etiology among all age groups.3,4 Risk factors include inadequate salivary flow, insufficient fluoride exposure, poor oral hygiene, and low socioeconomic status.5,6 Long-term consequences of untreated dental disease include pain, sepsis, disruption to family and work life, and an overall decrease in productivity.1,6 Considering the oral microbiota that contributes to caries is unique to each individual, universal or general caries prevention measures will not have the same efficacy for each patient.7,8
The demineralization process can potentially be reversed if it is detected and treated early. The mineral loss associated with disease leads to visual changes on the surface of the tooth, starting with white spot lesions and progressing to cavitation.8 The aim of incorporating fluoride therapies is to prevent lesion development or progression.
Sodium Fluoride
Fluoride varnishes were developed in the early 1970s to enhance contact time of the available fluoride with the surface of the tooth.9 Varnishes were approved by the United States Food and Drug Administration (FDA) in 1994 for use as cavity liners and treating dentinal hypersensitivity.9,10 Fluoride varnish application for caries prevention is considered an off-label use, but it has become the standard of care in dental practice.10
In clinical use, NaF varnish has a larger concentration of fluoride (22,600 ppm) than 12,300 ppm fluoride gel or 9,050 ppm foam.9 Varnish is effective in reversing incipient pit and fissure lesions, and evidence suggests it is particularly effective in primary teeth.11 Applying fluoride varnish two times to four times a year has been associated with a 43% decrease in decayed, missing, filled or extracted teeth in permanent dentition, and a 37% decrease in primary dentition.9
Silver Diamine Fluoride
Currently, two SDF concentrations, 12% and 38%, are used by US oral health professionals; these odorless and colorless solutions contain ammonium, silver, and fluoride ions.12 A systematic review by Tolba et al13 identified 38% SDF as generally being more effective than 12% SDF in arresting caries.
There are approximately 44,800 ppm fluoride ions within the 38% SDF solution. Seifo et al12 evaluated how DNA and proteins from bacteria in caries lesions are inhibited by silver and ammonia ions. Silver, ammonia, and fluoride ions found in 38% SDF solution assist in remineralization and arresting lesion progression. This material was first approved by the FDA in 2014 as a desensitizing agent.14 However, with new clinical evidence from off-label use, research suggests SDF may also have caries-arresting effects;14 consequently, the FDA granted “breakthrough therapy status” for its use in caries management.15
In 2021, Abdellatif et al16 conducted a randomized clinical trial comparing the effectiveness of 38% SDF and the atraumatic restorative technique (ART) for arresting caries in children. The ART clinical procedure used in this study included removing caries, as well as unsupported enamel and dentin, with a dental hatchet and excavator, prepping the site, and restoration using a glass ionomer cement. The results indicated 38% SDF was more effective in arresting lesions on the surfaces of primary teeth at both the 6-month and 12-month follow-up. Furthermore, application of 38% SDF was more efficient, requiring approximately 3 minutes, compared to approximately 14 minutes using ART chairside.16
While SDF offers a cost-effective and noninvasive way to arrest caries, the tradeoff is esthetics. A pediatric-based study by Crystal and Niederman17 focused on parental satisfaction and found that even with a 93% parental satisfaction with treatment efficacy, concerns were raised due to SDF’s permanent staining of affected tooth surfaces.
Fung et al18 sought to determine the optimal frequency of SDF application. Their randomized, controlled clinical trial compared the annual and biannual application of 12% SDF and 38% SDF on deciduous teeth. Results from the 30-month trial revealed that pediatric patients receiving the biannual application of 38% SDF had the most remineralized surfaces and arrested lesions compared to patients who received an annual application.18
Ammonium Hexafluorosilicate
An emerging formula that aims to arrest caries and treat dentinal hypersensitivity, AHF is not yet available for commercial use in the US. Due to it use of silica instead of silver, this formula does not stain caries lesions black, as does SDF. Suge et al19 evaluated dentinal tubule occlusion resulting from AHF’s calcium phosphate precipitation. Scanning electron microscopy was used to evaluate the agent’s occluding ability. The study showed that, after 7 days, the surfaces of the tubules were covered with newly formed calcium phosphate precipitate. While these results were not dependent on any specific concentration of AHF, the researchers found that a concentration of 1,000 ppm to 9,000 ppm AHF appeared optimal for tubule occlusion. Permeability of the dentin remained low throughout the experiment, which demonstrates that AHF can be beneficial for treating sensitivity.19
Hosoya et al20 observed color changes in the enamel, as well as the structure of the enamel, after AHF and 38% SDF application. The teeth used in this small in vitro study (n=20) were divided into four groups that varied by AHF application and artificial saliva immersion. The enamel and dentin were demineralized in a solution of 10% ethylenediaminetetraacetic acid, followed by an application of 35% phosphoric acid gel and soaking in the AHF solution. The treatment did not lead to staining; however, the ratio of calcium to phosphate was higher on the dentinal surfaces after AHF application without immersion in artificial saliva.20
Song et al21 studied whether AHF had cytotoxic effects on the tissues of the oral cavity. The impacts of various concentrations and durations of AHF exposure were examined on human gingival fibroblasts (hGFs). Concentrations of AHF used included 0.001%, 0.01%, 0.1%, and 1%. The agent was applied to cell cultures at 1 minute, 5 minutes, 10 minutes, and 30 minutes, and evaluated using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay to determine cell viability. The results demonstrated a reduced amount of viable cells on the treated hGFs with the various concentrations of AHF, compared to their respective control groups. The hGFs treated with the varying concentrations of AHF at only 1 minute did not show a significant difference in cell viability.21
In addition, Suge et al19 applied AHF for 3 minutes in their 2018 study. Once the AHF solution had been applied, the team used distilled water to rinse the tooth’s surface. Various solutions were evaluated to determine the most effective concentration, including 100 ppm, 1,000 ppm, 3,000 ppm, 5,000 ppm, 9,000 ppm, 11,000 ppm, 13,000 ppm, 15,000 ppm and 19,400 ppm. The results indicated that a 9,000 ppm AHF solution was the optimal concentration for both hypersensitivity treatment and remineralization.19 However, further research is required to determine the most effective frequency of clinical application.
Nano Silver Fluoride
Another emerging solution, NSF, has been studied for caries management.22 This formula contains nano silver particles, chitosan, and fluoride. It is believed to have antibacterial properties and act as an anti-caries agent. The size of the silver nanoparticles reduces the probability of permanent dark staining of the lesion, as occurs with SDF.22
Targino et al23 compared the antimicrobial qualities of 0.11 mol NSF, 38% SDF, and 0.12 chlorhexidine against S. mutans. Minimum bactericidal concentration (MBC) and minimum inhibitory concentration (MIC) were used to evaluate the extent to which the various solutions inhibited bacterial growth on agar plates. Results identified that 0.11 mol NSF and 38% SDF inhibited S. mutans at significant MBC and MIC levels, which would be vital to preventing and arresting caries.23
 In 2019, Gultom et al24 conducted an in vitro study to determine NSF’s ability to penetrate bacteria, disrupt cell division, and cause cell death. Several bacterial cultures of S. mutans were exposed to 38% SDF for a positive and negative control of unexposed S. mutans. The various plates were introduced to concentrations of NSF at 2.66%, 3.16%, 3.66%, and 4.16%. Results revealed that as the concentration of NSF increased, the viability of S. mutans bacterial growth decreased. The optimal concentration of NSF to inhibit bacterial growth was 4.16%. Each plate exposed to NSF showed lower S. mutans viability than the positive 38% SDF control plate. This demonstrated the effectiveness of 4.16% NSF in reducing the ability of S. mutans to adhere to a tooth’s surface, potentially preventing the formation of caries lesions.24
In 2019, Gultom et al24 conducted an in vitro study to determine NSF’s ability to penetrate bacteria, disrupt cell division, and cause cell death. Several bacterial cultures of S. mutans were exposed to 38% SDF for a positive and negative control of unexposed S. mutans. The various plates were introduced to concentrations of NSF at 2.66%, 3.16%, 3.66%, and 4.16%. Results revealed that as the concentration of NSF increased, the viability of S. mutans bacterial growth decreased. The optimal concentration of NSF to inhibit bacterial growth was 4.16%. Each plate exposed to NSF showed lower S. mutans viability than the positive 38% SDF control plate. This demonstrated the effectiveness of 4.16% NSF in reducing the ability of S. mutans to adhere to a tooth’s surface, potentially preventing the formation of caries lesions.24
In a randomized clinical trial, Burns and Hollands25 evaluated the application frequency and amount of NSF needed to produce optimal remineralization and caries-arresting results. The agent was applied to deciduous teeth with active lesions after using deionized water to rinse the tooth, cotton rolls to isolate the tooth, and a microbrush for surface application of NSF. Trial results determined that annual application of two drops of NSF applied over a 2-minute period allowed for optimum remineralization and lesion arrest.25
Along with its bactericidal and antimicrobial properties, NSF presents limitations due to its cytotoxic components. Sahu et al26 evaluated this agent’s cytotoxic effects on liver and colon cells in the human body. Both the HepG2 liver cells and Caco2 colon cells were exposed to 20-nm silver particles. Results showed DNA damage, as well as cellular oxidative stress in HepG2 cells. The authors also determined that liver cells are targets for nanosilver cytotoxicity and could be used to monitor toxic levels of NSF.26
Researchers have also examined fluoride’s possible effects on male reproduction.27 The investigators determined that fluoride can cause changes in the functional and structural behavior of spermatozoa, disruption of spermatogenesis, and disturbances of multiple hormone systems that impact male reproduction. Spermatozoa changes occur due to oxidative damage. Spermatogenesis is interrupted when levels of epidermal growth factor and epidermal growth factor receptors are depressed. The study also showed that fluoride can hamper reproduction by interfering with thyroid function. With considerations of liver and reproductive effects, further research is needed to determine the safe, yet effective, levels of nanosilver particles appropriate for all age groups.26,27
Conclusion
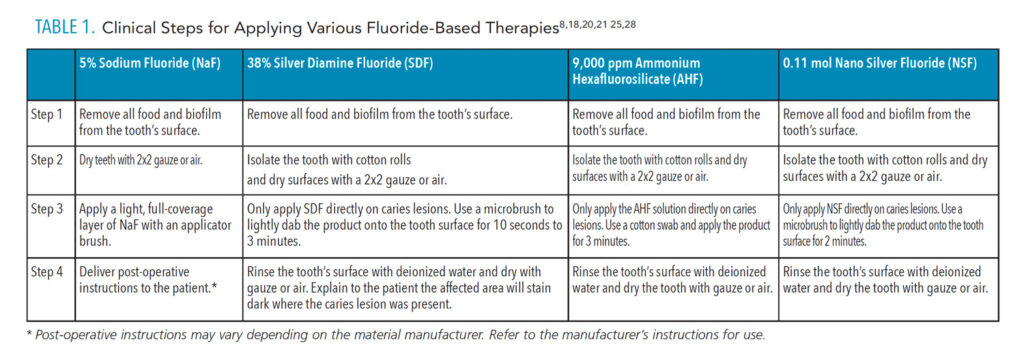
The clinical application process and frequency of application may differ between each fluoride therapy. Table 1 summarizes professional application of 5% NaF, 38% SDF, 9,000 ppm AHF, and 0.11 mol NSF. Application time can vary depending on patient compliance and behavior.18,25,28
While each patient’s caries experience is unique, lesions form as a result of genetic factors, environmental factors, host factors, and nutrition. Multiple fluoride-based therapies exist for preventing and managing caries and dentinal hypersensitivity. Offering bactericidal and remineralization properties, these agents have all shown to be effective in deciduous and permanent teeth. In clinical practice, evaluating each patient’s context can help determine which fluoride formula might be best suited for that individual.
Acknowledgement: The authors thank Taylor Derrig, RDH, BSDH, for her contributions to this manuscript.
References
- Griffin SO, Thornton-Evans G, Wei L, Griffin PM. Disparities in dental use and untreated caries prevalence by income. JDR Clin Trans Res. 2021;6:234–241.
- Wright JT. The burden and management of dental caries in older children. Pediatr Clin North Am. 2018;65:955–963.
- Lemos JA, Palmer SR, Zeng L, et al. The biology of Streptococcus mutans. Microbiol Spectr. 2019;7:10.
- Hernández-Sierra JF, Ruiz F, Pena DC, et al. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine. 2008;4:237–240.
- Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369(9555):51–59.
- Peres MA, Macpherson LM, Weyant RJ, et al. Oral diseases: a global public health challenge. Lancet. 2019;394:249–260.
- Grigalauskienė R, Slabšinskienė E, Vasiliauskienė I. Biological approach of dental caries management. Stomatologija. 2015;17:107–112.
- Costa e Silva AV, Teixeira JA, Melo Júnior PC, et al. Remineralizing potential of nano silver fluoride for tooth enamel: an optical coherence tomography analysis. Pesqui Bras Odontopediatria Clín Integr. 2019;19:e4002.
- Marinho VC, Worthington HV, Walsh T, Clarkson JE. Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev. 2013;7:CD002279.
- Bawden JW. Fluoride varnish: a useful new tool for public health dentistry. J Public Health Dent. 1998;58:266–269.
- Autio-Gold JT, Courts F. Assessing the effect of fluoride varnish on early enamel caries lesions in the primary dentition. J Am Dent Assoc. 2001;132:1247–1253.
- Seifo N, Robertson M, MacLean J, et al. The use of silver diamine fluoride (SDF) in dental practice. Br Dent J. 2020;228:75–81.
- Tolba ZO, Hamza HS, Moheb DM, Hassanein HE, El Sayed HM. Effectiveness of two concentrations 12% versus 38% of silver diamine fluoride in arresting cavitated dentin caries among children: a systematic review. Gaz Egypt Paediatr Assoc. 2019;67:1.
- Association of State and Territorial Dental Directors. Silver Diamine Fluoride (SDF) Fact Sheet. Available at: https://www.astdd.org/www/docs/sdf-fact-sheet-09-07-2017.pdf. Accessed October 19, 2021.
- Horst JA. Silver fluoride as a treatment for dental caries. Adv Dent Res. 2018;29:135–140.
- Abdellatif HM, Ali AM, Baghdady SI, ElKateb MA. Caries arrest effectiveness of silver diamine fluoride compared to alternative restorative technique: randomized clinical trial. Eur Arch Paediatr Dent. 2021;22:575–585.
- Crystal YO, Niederman R. Silver diamine fluoride treatment considerations in children’s caries management. Pediatr Dent. 2016;38:466–471.
- Fung MH, Duangthip D, Wong MC, Lo EC, Chu CH. Randomized clinical trial of 12% and 38% silver diamine fluoride treatment. J Dent Res. 2018;97:171–178.
- Suge T, Shibata S, Ishikawa K, Matsuo T. Fluoride activity of antibacterial ammonium hexafluorosilicate solution for the prevention of dentin caries. Am J Dent. 2018;31:103–106.
- Hosoya Y, Tadokoro K, Otani H, et al. Effect of ammonium hexafluorosilicate application for arresting caries treatment on demineralized primary tooth enamel. J Oral Sci. 2013;55:115–121.
- Song DX, Zheng LW, Shen SM, Chen XM. Cytotoxicity of ammonium hexafluorosilicate on human gingival fibroblasts. Toxicol In Vitro. 2013;27:2149–2155.
- Nanda KJ, Naik S. An in-vitro comparative evaluation of pre-treatment with nano-silver fluoride on inhibiting secondary caries at tooth restoration interface. Cureus. 2020;12:e7934.
- Targino AG, Flores MA, dos Santos Junior VE, et al. An innovative approach to treating dental decay in children. A new anticaries agent. J Mater Sci Mater Med. 2014;25:2041–2047.
- Gultom FP, Khoirunnisa N, Sahlan M, Soekanto SA. Evaluation of the potential of nano silver fluoride against Streptococcus mutans and Enterobacter faecalis in various stages of biofilm maturation. AIP Conference Proceedings. 2019;2092:030007.
- Burns J, Hollands K. Nano silver fluoride for preventing caries. Evid Based Dent. 2015;16:8–9.
- Sahu SC, Zheng J, Graham L, et al. Comparative cytotoxicity of nanosilver in human liver HepG2 and colon Caco2 cells in culture. J Appl Toxicol. 2014;34:1155–1166.
- Hu L, Ying J, Mu L, Yu SI, Liang Z, Clinch C. Fluoride toxicity in the male reproductive system. Fluoride. 2009;42:260–276.
- Crystal YO, Niederman R. Evidence-based dentistry update on silver diamine fluoride. Dent Clin North Am. 2019;63:45–68.
From Dimensions of Dental Hygiene. December 2021;19(12)36-39.



