 VALERIY_G/ISTOCK/GETTY IMAGES PLUS
VALERIY_G/ISTOCK/GETTY IMAGES PLUS
Ensure Infection Control Compliance With Chemical and Biological Indicators
Proper sterilization monitoring processes include a combination of mechanical, chemical, and biological indicators.
This course was published in the August 2021 issue and expires August 2024. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Identify the regulatory guidance agencies for infection control in dentistry.
- Discuss the instrument processing cycle.
- Describe the types of sterilizers and monitoring processes.
- Explain the best practices for chemical indicators and biological indicators.
While oral health professionals acquire baseline knowledge and meet clinical competencies related to infection control and prevention during their formal education, continuing education in this area is critical to providing safe dental care to patients. Safe dental visits encompass new COVID-19 protocols, as well as existing infection prevention measures, including verification of sterile instruments. The United States Centers for Disease Control and Prevention (CDC) and the Occupational Safety and Health Administration (OSHA) require verification of dental instrument sterility. Proper sterilization monitoring processes include a combination of mechanical, chemical, and biological indicators.1,2 This paper focuses on sterilization, including chemical indicators (CI) and biological indicators (BI).
OSHA is a division of the US Department of Labor, which has been protecting the safety of workers since 1971 through the use of mandatory regulations, known as standards. Employers must comply with OSHA standards. The Bloodborne Pathogens (BBP) Standard CFR 1910.1030 (exposure to BBP such as hepatitis B and C and human immunodeficiency virus), Hazard Communication (HAZCOM) Standard CFR 1910.1200 (safety with chemicals), and the Respiratory Protection Standard CFR 1910.134 are the OSHA standards used in dentistry.3,4 OSHA is a federal regulatory body that can investigate and impose fines for lack of compliance with standards.5,6 In 2020, the two most commonly violated OSHA standards in dentistry were the BBP and the Respiratory Protection Standard, which accounted for 68% of all cited standards.7
The 2003 CDC “Guidelines for Infection Control in Dental Health-Care Settings” are the most comprehensive guidance for the safe provision of dental treatment.1 An updated summary with checklists was released in March 2016.8 The CDC is an advisory body that provides evidence-based recommendations but cannot impose fines or enforce its own regulations, unless specific state boards adopt CDC guidelines, which then become state law. However, CDC recommendations can be enforced when they are related or connected to an OSHA regulatory standard, such as the BBP Standard.
Interim guidance from the CDC was published in response to the COVID-19 pandemic on May 28, 2020, to supplement the 2003 guidelines, and has been updated multiple times.9 Major highlights or procedural changes/recommendations for dentistry include social distancing, universal source control, prescreening protocols, personal protective equipment, aerosol reduction, and environmental controls.9
The CDC has not issued any new guidance regarding the instrument reprocessing cycle.9 As OSHA standards and CDC guidance remain the same for instrument processing, keep in mind that these are minimal requirements.
Instrument Processing Cycle
A proper sequence of instrument processing should be followed as outlined by the CDC, including safe transportation; receiving, cleaning, and decontamination; preparation and packaging; sterilization; and storage.1 The CDC recommends safely transporting instruments from the operatory to the sterilization area in a hard-walled container to avoid percutaneous injury.1 Instrument reprocessing is a multistep process that includes cleaning (via ultrasonic, washer, or washer/disinfector including rinsing and drying); packaging (use of pouches with built-in chemical indicators or placement of chemical indicators inside of wrapped cassettes); sterilization; and storage.1
The CDC requires heat sterilization for all critical and semi-critical items in compliance with the OSHA BBP Standard.1,2 Monitoring of the sterilization process using physical (watching gauges for time, temperature, and pressure); chemical (color changes on pouches or internal indicators to verify all sterilization parameters are met); and biological monitoring (regular spore testing with BIs) as required by CDC is critical to ensure proper sterilization parameters are reached.1
Spore testing is required weekly.1 Again, CDC requirements are minimal standards. Best practices may include performing biological monitoring more frequently based on practice needs. High-volume practices may need to perform biological monitoring more frequently. New ultra-rapid technology that offers 20 minute in-office BI monitoring systems may help clinicians exceed minimal standards. Performing daily monitoring in-office could be time efficient and exceeds current BI monitoring standards.
Regulatory Guidance for Sterilization
Proper sterilization monitoring processes include a combination of mechanical, chemical, and biological indicators. Several infection control recommendations related to CI and BI are outlined in the comprehensive 2003 CDC guidelines, which remain the gold standard in dentistry.1 CDC guidance is broad, allowing for flexibility in the implementation of its recommendations. Dental practices implement CDC recommendations in myriad ways, as CDC does not provide specific instructions as to how to carry out infection controls tasks. This broad guidance allows oral health professionals flexibility in the implementation based on the setting. However, this flexibility results in little standardization in dentistry as compared to medicine. There is very little oversight unless infection control breaches are reported.
For healthcare facilities, the Association for the Advancement of Medical Instrumentation (AAMI) provides comprehensive guidance for instrument processing and steam sterilization activities, regardless of the size of the sterilizer or the facility, and provides a resource for all personnel who use steam sterilization.10 AAMI ST79 is a comprehensive guidance document used in healthcare facilities and provides standardization and oversight for infection control tasks. AAMI ST79 offers guidance for the operation of sterilizers; standards for decontamination/instrument processing; criteria for steam sterilization; physical standards for instrument processing areas including sterile storage, staff qualifications, and step-by-step reprocessing guidance; and care and maintenance of all types of steam sterilizers, including for the small tabletop sterilizers frequently used in dentistry.10
Ensuring that sterilizers are functioning properly is critical for safe practice and eliminating the spread of disease. External accrediting bodies in healthcare use AAMI ST79 to ensure standardization of sterilization procedures, providing external oversight and reporting. Although AAMI ST79 is not required in dentistry, it is an excellent supplement to OSHA and CDC guidance.
Types of Sterilizers
Autoclaves are US Food and Drug Administration (FDA)-cleared medical devices that use steam under pressure, and they are the most frequently used sterilizers in dentistry. There are two common types of autoclaves: gravity displacement (Class N) and dynamic air removal (Class S and B).1 Gravity displacement units are the most frequently used in dentistry. These sterilizers admit air at the top or side of the chamber and, because steam is lighter than air, gravity forces the heavier air out, exiting through the bottom drain. This passive method requires more drying time. Trapped air or cool pockets can be problematic with gravity displacement sterilizers, so placement of the BI is critical.
Dynamic air removal sterilizers—such as Class S and Class B—use a vacuum pump to remove all the air from the chamber before steam is emitted. The advantages of class S and B include the nearly instantaneous steam penetration, shorter drying time, and ability to fully penetrate even the smallest hollow tubing of medical instruments. No matter which type of sterilizer, it is critical to let the load cool and dry fully to avoid wicking microorganism into the contents. Wicking is a process that allows bacteria or other contaminants to penetrate into the inside contents of a sterilization pouch or pack.
Widely used in Europe and Canada, the European standard EN 13060 is a strict guideline that defines requirements for small steam sterilizers. Class B sterilizers meet this standard. Although the CDC does not require Class B autoclaves, it does provide high levels of sterility assurance that meets the EN 13060 hollow tube test.10 Manufacturers offer a wide range of autoclaves for clinicians looking to enhance their infection control practices by using class B technology.
Some manufacturers offer the latest sterilization technology, including autoclaves that provide the following benefits: upgradeable from a Class S to a Class B with a simple software upgrade, fast cycles completed in 32 minutes, a variety of documentation and traceability options and barcodes, and smart technology. Class B autoclave meets high standards of sterilization. Additional new technology includes a class B autoclave with a “pretreatment” cycle that addresses the air in the chamber before the preliminary air removal stage, ensuring the air released back into the ambient air is free from microorganisms and viruses. This technology might provide additional peace of mind. Manufacturers have produced autoclaves that meet the need with faster cycles that deliver dry instruments without wicking. This technology provides closed-door drying (instruments dry without having to monitor and open the door)—allowing more cycles throughout the day—and an automatic safety shut off.
Sterilization Monitoring
Guidance from CDC and OSHA requires the use of chemical and biological monitoring to ensure sterilization parameters are achieved.1,2 Chemical monitoring is based on the use of indicators. CIs use sensitive material to detect physical conditions, such as exposure time and temperature. These can be external or internal indicators that might change color when exposed to a sterilization process. Prior to sterilization, during preparation and packaging, CIs are added to monitor a variety of internal and external parameters to determine if steam has reached the contents inside.10
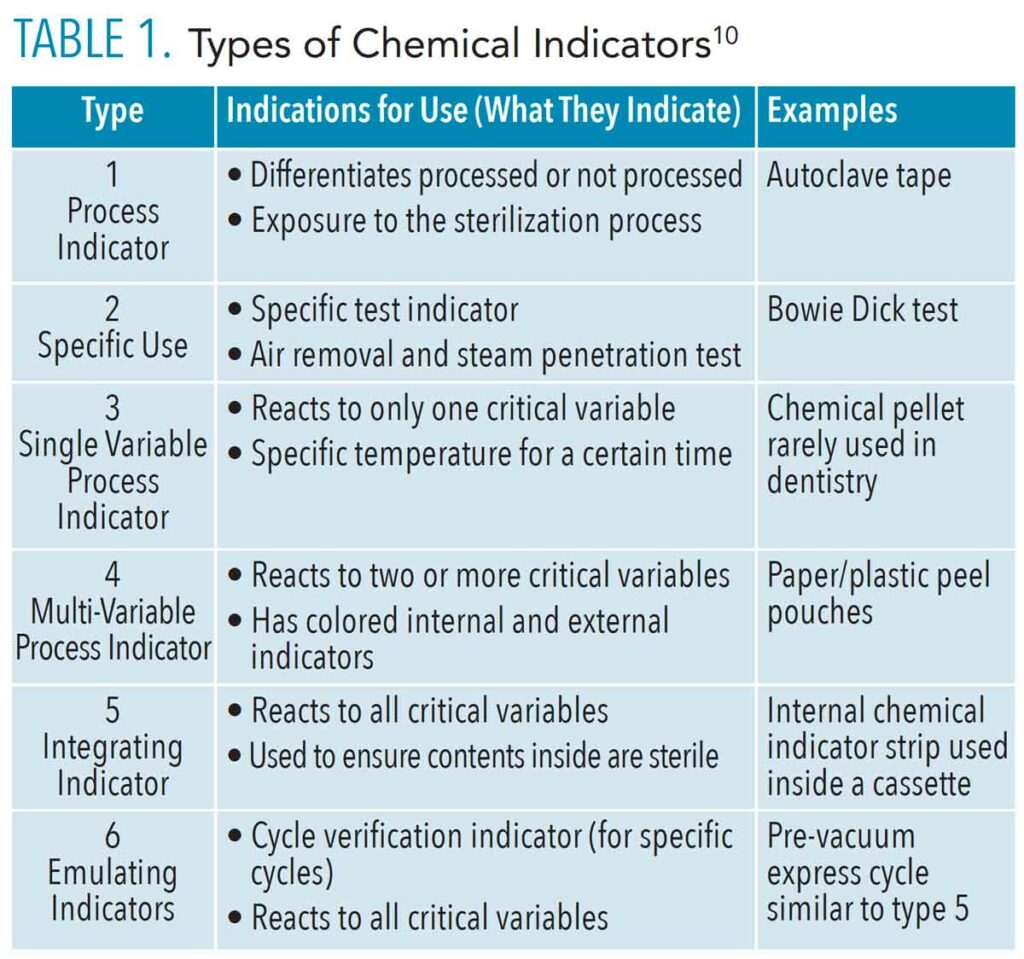
There are six types of CIs according to AAMI ST 79 (Table 1).10 Type 1 is the most basic and demonstrates that the item has been exposed to one sterilization process (such as heat) and distinguishes between processed and nonprocessed items. Type 1 CIs, such as autoclave tape used to secure wrapped cassettes, measure a single parameter.
Type 2 is a specialty indicator used for specific tests such as the Bowie Dick test, which measures the air-removal performance in prevacuum steam sterilizers. These indicators test for the presence of air in the steam sterilizing chamber. A positive test indicates air leakage, which inhibits the conditions necessary for sterilization. This test is performed at the start of the day to ensure the autoclave is functioning optimally.
Type 3 is a single parameter indicator designed to respond to only one variable of the sterilization process, such as time or temperature. This type is not recognized by the FDA.
Type 4 indicators are more accurate. They react to two or more critical parameters of the sterilization process (such as time and temperature) and indicate exposure to the sterilization cycle. Pouches are a great example of type 4, as they have internal and external markers that change color when exposed to the parameters. Pouches must be examined after sterilization to ensure the parameters were met (color change). There is debate about placement of pouches in the autoclave; whether paper side up or plastic side up. It is ideal to stack pouches side to side in a pouch rack for better steam penetration,1,10 but it is always best to consult the manufacturer’s IFU on proper placement without a rack.
Type 5 integrating indicators are designed to react to all of the critical variables of the specific sterilization process (time, temperature, and pressure). They are similar to BIs, but the type 5 integrating indicator does not contain live bacterial spores and should not be used as a substitute for a BI. Integrating indicators may be used as internal chemical indicators, and process challenge devices to monitor and release nonimplant loads. In certain situations, the results of the type 5 can be used to release an implant before the BI result is available. Type 5 integrators are used inside a cassette to ensure the contents inside are sterile.
Type 6 emulating indicators are designed to react to all critical variables of a specified sterilization cycle. They should only be used in the specific cycle for which they are labeled. For example, one of the available Type 6 CI is labeled for use in a prevacuum, express cycle. This indicator can be used only in that cycle. Type 6 CIs do not contain spores and are not substitutes for BIs. They can be used to release implant loads while waiting for biological spore test.10
Best practices with CIs depend on the type of packaging used. If pouches are used, they should meet the following requirements:1 type 4, compatible with the autoclave, and visibly labeled with nontoxic ink or pencil on the nonporous side with the date of sterilization, the sterilizer number, and the cycle number to aid in traceability. If cassettes are used, type 5 integrating indicators must be inside each cassette, wrapped using FDA-approved wrap, and secured with a type 1 tape.1 Logging all activity related to sterilization is another important consideration. The CDC recommends keeping a log that contains information related to mechanical, chemical, and biological monitoring (Table 2).1

Biological Indicators
BIs, also known as “spore-tests,” are used to monitor the functioning of autoclaves by testing for the inactivation of highly resistant microorganisms.1 An inactivated BI indicates pathogens in the load have been killed, thus verifying “sterilization.” Performing BI is the only way to ensure that sterility has been achieved.1
Two methods of BI testing are available: in-office incubation and mail-in services.1 Mail-in services are easy to use and inexpensive. A BI strip is placed in an autoclave cycle and then sent by mail for incubation and results. Correct placement of the BI in the autoclave is critical to ensure steam has penetrated the most challenging area of the sterilizer. It is best to place the BI in the center of the load, as this is where cool air pockets exist in gravity displacement sterilizers.1,10 It is best to place the BI near the door in class S or class B (dynamic air removal sterilizers).10 Notification of failed tests might take longer due to delays in mail delivery. By the time of a failed spore test notification, several loads of instruments may have been run and may not be sterile. Tracking and retrieval of these instruments might be more difficult. Mail-in methods may be adequate for lower volume practices. Table 3 outlines troubleshooting tips with BI.
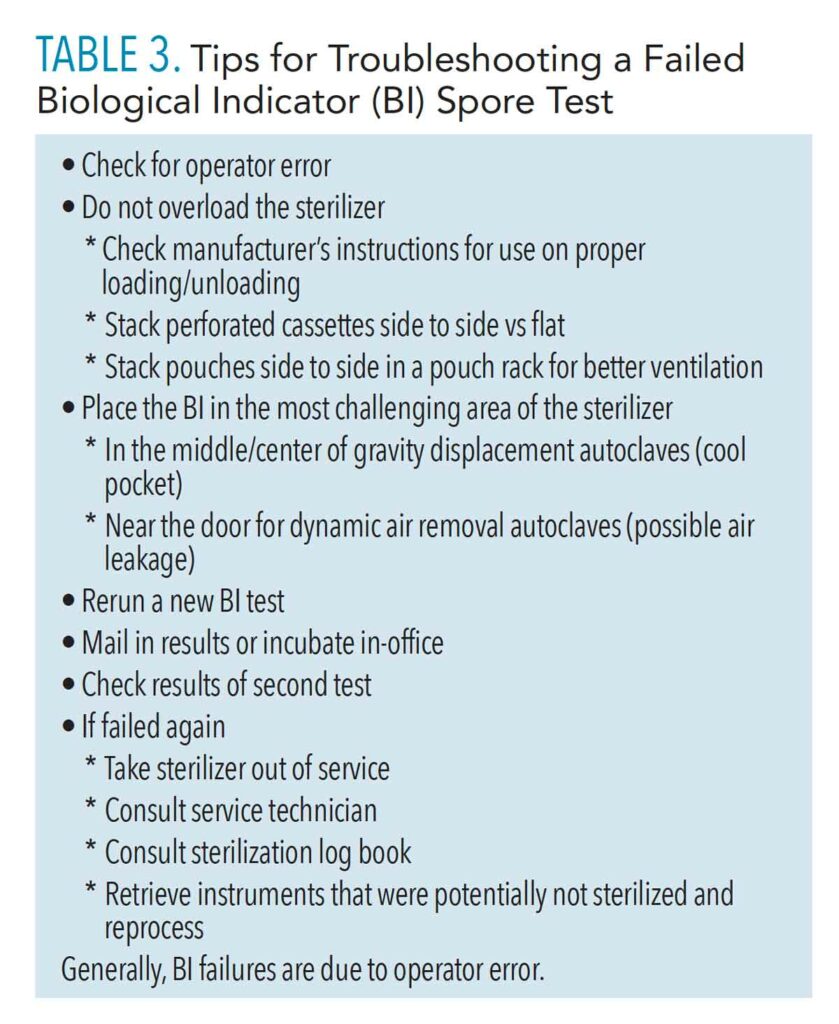
In-office incubation methods of BI may be preferred to ensure verification of sterility in a timelier manner (less than 24 hours); however, the up-front cost may be more expensive. In this process, a test vial is placed and run in a regular autoclave cycle, is incubated for specified time period, and then a color change is compared. If a test fails, a new test must be done. Most BI test failures are due to operator error such as overloading the sterilizer, improperly loading cassettes and pouches, and incorrect BI placement. Truly failed spore tests can be quicker to flag, retrieve, and log with in-office incubation methods.
Advantages of in-office incubation include rapid nature of results, printouts of results, ability to test several vials at a time from different types of autoclaves, and ability to test more frequently than the CDC recommends. The CDC recommends performing BI at least weekly.1 The risks of performing BI on a minimal weekly basis includes the potential for disease transmission and inability to retrieve potentially unsterile instruments in a timely fashion. Best practices may include performing BI more frequently, such as daily or multiple times per day depending on the practice volume and types of loads.
Conclusion and Overall Best Practices
Guidance from the CDC and OSHA requires the use of chemical and biological monitoring to ensure sterilization parameters are achieved.1,2 The importance of regular BI testing is critical for the provision of safe care. Oral health professionals must ensure safe dental visits by adhering to current infection control guidance. Exceeding minimal standards may be considered best practice. Higher volume practices may need to perform BI more frequently. Dentistry has had to adapt to evolving infection control challenges during the COVID-19 pandemic. Oversight mechanisms in the medical profession—such as the use of AAMI/ANSI ST 79 by external accrediting bodies to ensure infection prevention measures are in place—may need to be considered for dentistry.
References
- Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep. 2003;52:1–66.
- Occupational Safety and Health Administration. Bloodborne Pathogens Standard 1910.1030 at: click here. Accessed July 23, 2021.
- Occupational Health and Safety Administration. Hazard Communication 1910.1200 Standard. Available at: click here. Accessed July 23, 2021.
- Occupational Health and Safety Administration. Respiratory Protection Standard 1910.134. Available at: click here. Accessed July 23, 2021.
- Occupational Safety and Health Administration Enforcement. Available at: click here. Accessed July 23, 2021.
- Occupational Safety and Health Administration. Fine Fact Sheet. Available at: click here. Accessed July 23, 2021.
- Occupational Safety and Health Administration. Commonly Cited Standards. Available at: click here. Accessed July 23, 2021.
- United States Centers for Disease Control and Prevention. Summary of Infection Prevention Practices in Dental Settings: Basic Expectations for Safe Care. Available at: click here. Accessed July 23, 2021.
- United States Centers for Disease Control and Prevention. Guidance for Dental Settings. Interim Infection Prevention and Control Guidance for Dental Settings During the COVID-19 Response. Available at: click here. Accessed July 23, 2021.
- Association for the Advancement of Medical Instrumentation. ANSI/AAMI ST-79. Comprehensive Guide to Steam Sterilization and Sterility Assurance in Health Care Facilities. Available at: click here. Accessed July 23, 2021.
From Dimensions of Dental Hygiene. August 2021;19(8):26-28, 31.



