Oral Cancer Detection
Dental hygienists play a unique role in early oral cancer detection by routinely providing examinations and educating patients about self-examination and risk factors.
This course was published in the August 2016 issue and expires August 2019. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the demographics of patient populations most likely to develop oral cancer.
- Identify the risk factors for cancers of the head and neck.
- Demonstrate the appropriate method to clinically examine the oral cavity for precancerous and cancerous lesions.
- Distinguish the clinical characteristics of noncancerous, precancerous, and cancerous lesions.
The American Cancer Society estimates that more than 48,000 people will develop oral and oropharyngeal cancer in 2016 and that over 9,500 will die of this disease.1 Despite the fact that most of these cancers are readily visible, and therefore should be easily detectable and treatable at an early stage, the 5-year survival rate has not changed significantly over the past several decades, remaining at about 50% to 55%.1 The responsibility for the lack of early detection and improvement of the 5-year cure rate can be shared equally by patients and health professionals. From the patient’s standpoint, some oral cancers are asymptomatic or produce symptoms similar to other oral conditions and therefore do not trigger an urgent response. Also, some patients fail to seek professional dental care on a regular basis. Among health professionals, there is often a failure to perform an adequate oral examination or an inability to recognize early precancerous or cancerous lesions.
The American Cancer Society reports that oral cancer most commonly occurs in individuals age 55 or older. However, one quarter of oral cancer patients are younger than 50, and this proportion is increasing.1 While the reason for this trend is unclear, human papillomavirus (HPV) infection—particularly HPV 16 and HPV 18—has been implicated.2 Changing demographics mean that all patients, regardless of age, require the same attention. Men are affected by oral cancer twice as often as women, and the disease is more prevalent among blacks compared with whites.3 Moreover, blacks appear to develop oral cancer at younger ages than whites.3
When performing a through head and neck examination at each appointment, dental hygienists should be aware that the tongue, particularly the lateral border; floor of the mouth; gingiva; palate; and lips are among the most common sites for oral cancer. Cancer, however, can develop in any tissue in the oral cavity or head and neck region; therefore, no area should be overlooked in the examination. Palpation of the neck is also important because cancers in the oral cavity can spread to the submandibular and cervical lymph nodes.
RISK FACTORS
Understanding the risk factors for oral cancer will help identify patients who require more frequent follow-ups. A strong association between tobacco and alcohol use and oral cancer exists. Studies have shown that the risk of oral cancer is up to nine times greater for smokers than nonsmokers and up to 17 times greater for heavy smokers.2 In addition to smoking, the use of snuff, chewing tobacco, and betel quid have also been associated with an increased risk of oral cancer. Patients unwilling to quit should be told to position snuff or chewing tobacco at different sites around the mouth instead of consistently positioning it in the same place. Dental hygienists should provide patients with information regarding tobacco cessation programs regardless of what type of tobacco is used.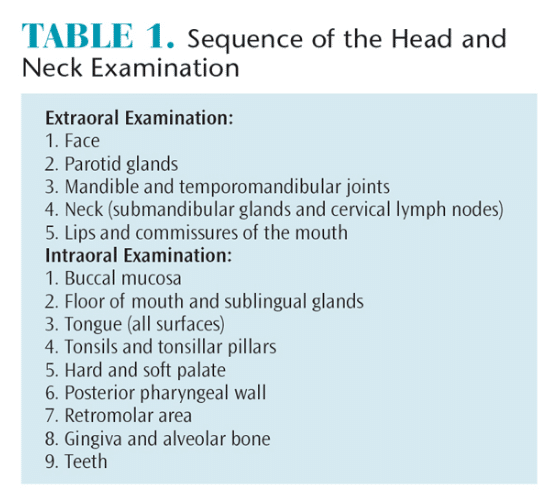
Alcohol consumption is a major risk factor for oral cancer, with moderate-to-heavy drinkers experiencing a three- to nine-fold higher risk of developing oral and pharyngeal cancer than nondrinkers. Combining heavy drinking and smoking increases oral cancer risk, with these individuals experiencing a 38% greater risk for developing cancer than nonsmokers/nondrinkers.4 Abstaining from alcohol appears to reduce the risk of oral and pharyngeal cancer, but this effect doesn’t appear for at least 10 years following cessation.4
Several studies have linked an increased risk of oral cancer to diets low in fruits and vegetables. Furthermore, studies have shown that a diet containing adequate amounts of fruits and vegetables is associated with a reduced oral cancer risk.5 However, because heavy drinkers often have poor nutritional habits, diet may not be a contributing factor on its own. Nevertheless, dental hygienists should periodically evaluate patients’ diets using a dietary assessment questionnaire and provide nutritional counseling if deficiencies are found.6
Chronic exposure to sunlight (ultraviolet light) is a risk factor for cancer of the face and lip. It is most often seen among those with outdoor jobs in which they are exposed to direct sunlight for long periods. The incidence is higher among men than women. During extraoral examinations, if signs of overexposure to the sun are noticed, dental hygienists should educate patients on proper lip and facial protection. Tactics include applying sunscreen with a minimum skin protective factor of 15 and wearing a wide-brimmed hat.7
Studies have also shown an association between oral HPV infection and oral cancer. HPV-16 has been found in up to 22% of oral cancer cases and HPV-18 has been found in up to 14% of oral cancer cases.8 Oral sex may be one of the factors responsible for the increased incidence of oral cancer in young cohorts.9,10 To decrease the incidence of such cancers, dental hygienists should educate their patients, or the parents of their patients, regarding vaccination for HPV-16 and HPV-18.
DETECTION OF ORAL CANCER
The most important steps in oral cancer detection include educating patients on the importance of periodic extra- and intraoral examinations, as well as teaching patients how to conduct self-examinations. Careful visual and digital examination, which is part of a patient’s general evaluation, should be done in a systematic sequence in order to ensure thoroughness. Table 1 describes a suggested examination sequence. After extraoral sites have been examined, the anterior part of the mouth should be evaluated and finally the posterior oral and pharyngeal structures. This sequence allows patients to grow accustomed to the exam and may increase compliance.
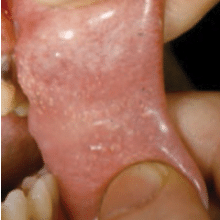
Table 2 lists the common signs that may indicate the presence of a malignancy. In simplest terms, any deviation from the norm is suspect. Loose teeth may be frequently encountered, but if they are limited to an isolated area and there is no gingival or periodontal involvement, cancer should be considered as a cause. Likewise, unexplained gingival bleeding, which may be a sign of leukemia or other systemic conditions that necessitate prompt referral to a patient’s primary care provider, should be noted as suspicious.
Patients should be asked during the medical history review about the presence of unexplained facial tingling or numbness; difficulty chewing, swallowing, or moving the jaw or tongue; hoarseness; chronic sore throat; and change in voice.
Because ordinary clinical examination may fail to detect very precancerous or cancerous lesions, ongoing effort has been dedicated to developing noninvasive, adjunctive diagnostic aids. The most common diagnostic tools are the use of vital staining with toluidine blue dye, the use of fluorescent light or blue spectrum light to note abnormal changes in the oral mucosa, and computer-assisted transepithelial oral brush biopsy.
Toluidine blue dye is applied to the involved mucosa. Dark blue stained areas remaining after an oral rinse to remove mechanically retained dye are then subject to biopsy. It can be helpful in determining the areas of visible lesions to biopsy, but it does not detect early mucosal changes.
A fluorescent light can be used to help detect abnormal mucosal changes after the application of dilute acetic acid. However, no significant differences have been found in detection rates between the use of this system and ordinary incandescent light for conventional visual examination.11 A blue spectrum light that causes the normal oral mucosa to fluoresce is also used in oral cancer detection. When this pattern is altered, the area of difference is thought to be potentially malignant. However, this method may not be able to differentiate between dysplasia and benign inflammatory conditions.12
The brush biopsy acquires deep mucosal cells for computer-assisted microscopic examination. Although a positive result is very specific, a negative result does not necessarily rule out the presence of a precancerous or cancerous lesion.
Systematic reviews regarding the use of various adjunctive diagnostic tests have concluded that the quality of the studies that advocate their use is poor. These expert panels noted that careful clinical examination, surgical biopsy, and histological assessment remain the gold standard for diagnosis of oral cancers.13
A novel approach to screening for oral cancer assays salivary biomarkers. While this approach, when ultimately perfected, could potentially be used as a screening mechanism for the presence of oral cancer, it still would rely on examination by dental professionals to identify the actual location of the lesion.14 Another type of salivary test detects the presence of HPV, which can be related to oral cancer. Although the test can accurately determine viral presence, a positive test does not mean that cancer is present nor does a negative result assure oral cancer is not present.
DIAGNOSIS OF ORAL CANCER
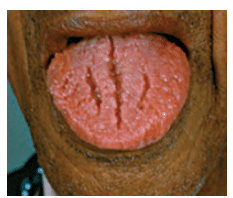
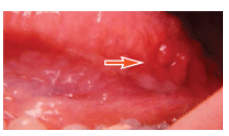
Oral lesions can be classified into three categories: noncancerous (benign), precancerous, or cancerous (malignant). Recognizing normal oral findings and developmental abnormalities that can resemble benign or malignant lesions is important. These conditions include maxillary and mandibular tori; Fordyce granules; ectopic sebaceous glands found in the buccal mucosa and lips (Figure 1); fissured or scrotal tongue (Figure 2); lingual tonsil (Figure 3A and Figure 3B); benign migratory glossitis or “geographic tongue” (Figure 4); lingual varicosities; and lingual thyroid.
The most common lesions of the oral cavity are inflammatory or traumatic lesions and benign tumors. Benign tumors can arise from the epithelium (eg, papilloma), connective tissue (eg, fibroma, lipoma), or bone (eg, osteoma). A papilloma is made up of small finger-like projections, giving it a verrucous or “cauliflower” appearance. Connective tissue tumors generally are soft and rounded, may be sessile or pedunculated, and are covered with normal mucosa. In contrast, an osteoma is a hard, exophytic lesion attached to the jaw.
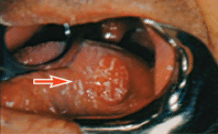
Inflammatory, traumatic, and ulcerative lesions are easily confused with oral malignancies. An aphthous ulcer is well circumscribed with a grayish central area surrounded by an erythematous halo, is painful, and tends to appear on nonattached mucosal surfaces, such as the mucosa of the cheek. A herpes simplex ulcer (fever blister, cold sore) is also painful; is usually larger than an aphthous ulcer; has a ragged border; and tends to appear on the attached mucosa of the gingivae, palate, or lips. Rapid onset, history of previous occurrences, and the presence of pain help in differentiating these ulcers—which should disappear in a few weeks—from oral cancer.
A traumatic ulcer can mimic the appearance of oral cancer, particularly when its presence is prolonged by a persistent source of irritation, such as a rough edge of a tooth or dental appliance, or the application of inappropriate medications, typically by the patient. Elimination of the suspected causes should result in healing within 1 week to 2 weeks. Otherwise, a biopsy is indicated.
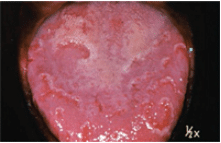
The most common precancerous lesion is leukoplakia, which has been described as a white patch on the mucosa that does not rub off and is not caused by any other disease. Leukoplakia is a response to chronic irritation from such sources as smoked or smokeless tobacco, alcohol abuse, or trauma. Clinically, it may range from a faint whiteness to a white, leathery, fissured appearance. There is also a speckled form of leukoplakia that has scattered red spots. There is little correlation between the clinical appearance of a lesion and its histology. This means that an innocuous-looking lesion may actually be cancer.
Lichen planus is a relatively common white lesion resembling leukoplakia. It generally has a reticular form characterized by interlacing white lines, producing a lacy appearance (Figure 5). However, it may also have an erosive or ulcerated form. Recognizing lichen planus is important because it is associated with hepatitis C15 and may undergo transformation to squamous cell carcinoma.16
Erythroplakia, also a precancerous lesion, refers to a red patch on the oral mucosa. It appears to have the same etiology as leukoplakia. Although less common than leukoplakia, erythroplakia is more likely to develop into a malignancy because it represents an inward rather than an outward proliferation of cells.
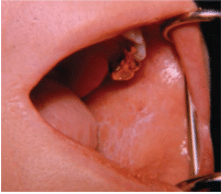
While all precancerous lesions have a predilection to develop into cancers, not all lesions will become cancerous. If possible, however, precancerous lesions should be removed. When this is not possible, the lesions should be subjected to regular follow-up evaluation and biopsy of suspicious areas.
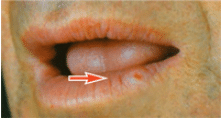
Oral cancer may appear as an ulcer, an exophytic lesion, or a submucosal induration (hardness) or mass with an intact mucosal covering. When palpated, a cancerous lesion is more likely to seem fixed in place rather than mobile inside the tissues. It may also be present in an area of leukoplakia or erythroplakia. Common sites are the lower lip, posterolateral aspect of the tongue, and the floor of the mouth. It occurs less frequently on the gingiva, buccal mucosa, and palate. A cancerous ulcer, which is usually painless (or only slightly painful), generally has a raised, irregular, thickened border with a hard consistency, which distinguishes it from the inflammatory ulcer that is painful when manipulated, softer, and less likely to appear exophytic (Figure 6). In the early stages, most oral cancers display a smooth reddish bottom that later becomes more granular in nature as it adopts a grayish-yellow appearance. Exophytic variants usually appear as firm reddish tumors with nodular or polypoid shapes.
Whenever a lesion is detected and the diagnosis is unclear, dental hygienists should record its location, size, shape, color, and texture, and take a photograph so that the site can be re-examined and subsequent changes can be noted. After the dentist has seen the patient, the patient should be referred immediately for scalpel biopsy or reappointed with the dentist for a follow-up examination in 2 weeks. If the lesion has not regressed or resolved in 2 weeks, a biopsy is indicated.
CONCLUSION
Oral cancer remains a major public health issue, regardless of age, race, or economic status. The early detection of oral cancer remains key to improving patient survival rates. This can only be accomplished through understanding of the common signs and symptoms of cancer and thorough clinical examination of the face, mouth, and neck at regular intervals. Dental hygienists play a unique role in early cancer detection by routinely providing such examinations and educating patients about self-examination and control of risk factors.
References
- American Cancer Society. Oral Cavity andOropharyngeal Cancer. Available at: cancer.org/cancer/oralcavityandoropharyngealcancer/detailedguide/oral-cavity-and-oropharyngeal-cancer-keystatistics. Accessed July 1, 2016.
- Blair EA. Head and Neck Carcinoma in the YoungPatient. Available at: emedicine.medscape.com/article/855871. Accessed July 1, 2016.
- Neville BW, Day TA. Oral cancer and precancerous lesions. CA Cancer J Clin. 2002;52:195–215.
- Oral Cancer Foundation. Risk Factors. Available at: oralcancerfoundation.org/cdc/cdc_chapter3.php.Accessed July 1, 2016.
- Llewellyn CD, Johnson NW, Warnakulasuriya S.Factors associated with delay in presentation among young patients with cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:707–713.
- Andersen LF, Johansson L, Solvoll K. Usefulness of a short food frequency questionnaire for screening of low fruit and vegetable and for intake of fat. EurJ Public Health. 2002;12:208–213.
- American Melanoma Foundation. Prevention.Available at: melanomafoundation.org/prevention/Prevention.htm. Accessed July 1, 2016.
- Sugerman PB, Shullitoe EJ. The high risk human papilloma viruses and oral cancer: Evidence for and against a causal relationship. Oral Dis.1997;3:130–147.
- Shiboski CH, Schmidt BL, Jordan RC. Tongue and tonsil carcinoma: increasing trends in the US population ages 20-44 years. Cancer.2005;103:1843–1849.
- Rettig E, Kiess AP, Fakhry C. The role of sexual behavior in head and neck cancer: implications forprevention and therapy. Expert Rev Anticancer Ther.2015;15:35-49.
- Oh E, Laskin DM. Efficacy of the ViziLite system in the identification of oral lesions. J Oral MaxillofacSurg. 2007;65;424–426.
- Rashid A, Warnkulasuriya S. The use of ligh tbased(optical) detection systems as adjuncts in the detection of oral cancer and oral potentially malignant disorders: a systematic review. J Oral Pathol Med. 2015;44:307–328.
- Macey R, Walsh T, Brocklehurst P, et al.Diagnostic tests for oral cancer and potentially malignant disorders in patients presenting with clinically evident lesions. Cochrane Database Syst Rev. May 29, 2015;5:CD010276.
- Cheng YL, Rees ST, Wright J. A review of researchon salivary biomarkers for oral cancer detection.Clin Transl Med. 2014;3:3–15.
- Lodi F, Pellicano R, Carrozzo M. Hepatitis C virusinfection and lichen planus: a systematic review with meta-analysis. Oral Dis. 2010;16:601–612.
- Fitzpatrick SG, Hirsch SA, Gordon SC. The malignant transformation of oral lichen planus and oral lichenoid lesions: a systematic review. J AmDent Assoc. 2014;145:45–56.
From Dimensions of Dental Hygiene. August 2016;14(08):60,63–65.




[…] numbers are a call to action for Evans who spreads the word about early oral cancer detection on social media, at dental conferences, and through daily encounters with fellow […]
[…] can be made in a dental setting during a conventional visual and tactile examination. Prompt oral cancer detection and diagnosis allows patients to begin appropriate therapy without […]