Tooth Whitening Safety
What the evidence says about the effects of bleaching on tooth structure.
Tooth whitening has been practiced for more than 100 years and is an effective way to enhance a smile’s esthetics.1 In a society that places great emphasis on appearance, the demand for brighter, whiter smiles has made bleaching one of the most popular dental procedures. The increased demand is accompanied by a wide scope of tooth whitening options—ranging from professional in-office procedures to professionally-dispensed home-use whiteners to over-the-counter products.
Regardless of the whitening method, the active ingredient in these material is almost exclusively hydrogen peroxide (HP) or carbamide peroxide. Whitening is a chemical oxidation process where oxygen and hydroxyl radicals released from HP react with the complex ring structure of stain molecules. The oxygen and hydroxyl radicals then oxidize and convert the stain molecules into more simple chain structures, thus altering the reflective index of the tooth, making the tooth appear lighter.2
The efficacy of peroxide-containing tooth whitening products has been well established,3 but concern over safety was initiated when at-home bleaching products were introduced.4 A primary concern is the effect of direct exposure of whitening agents on enamel. Several studies have shown that surface morphology alterations, 5–7 loss of mineral,8–13 and microhardness reduction can result.14,15
MORPHOLOGIC CHANGES IN THE ENAMEL
The equilibrium that exits between oral biofilm and apatite crystals at the enamel surface is a dynamic process involving a continuous ion exchange in both directions across the tooth surface to maintain proper mineral balance.16 This physiologic process in the oral cavity, however, makes it difficult to interpret the clinical relevance of laboratory measurements on enamel surface morphology changes, mineral loss, and microhardness changes.
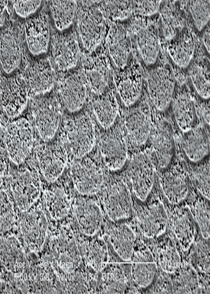
Profilometry and diverse microscopic techniques including scanning electron microscopy (SEM), confocal laser scanning microscopy, and atomic force microscopy have been adopted to observe and quantify changes in surface topography. Among the different microscopic techniques available, SEM is widely used in analyzing the surface morphology of enamel and dentin following whitening. SEM images have a large depth of field and yield high-resolution,
almost three-dimensional images (Figure 1). Varying results have been reported in studies that use SEM to determine tooth surface changes caused by whitening procedures. The results range from no changes17,18 to mild surface pitting and enamel porosity at localized areas to significant surface alterations.5–7 Study design, type and concentration of peroxide compound, pH, and exposure time can all affect study results. It is difficult to replicate the dynamic in vivo environment of the oral cavity in in vitro study protocols.
The use of products with particularly low pH can also affect results.19 A recent study comparing neutral and acidic HP on the surface morphology demonstrated that neutral HP did not affect the surface morphology whereas acidic HP resulted in significant enamel surface changes.20 Thus, studies that use whitening agents with relatively low pH may be describing demineralization effects caused by acidic erosion as opposed to HP.19
MINERAL LOSS
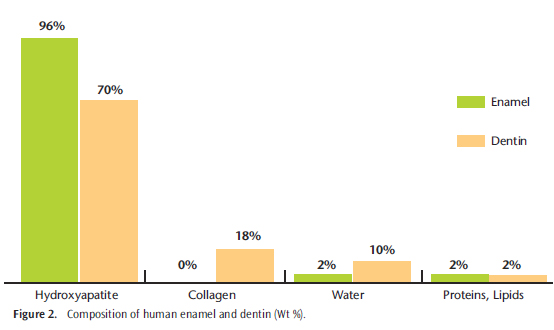
Fully formed enamel is the most mineralized matrix in the human body, consisting of approximately 96% minerals, 4% organic materials, and water (Figure 2).21 This high mineral content renders enamel susceptible to dissolution when the pH drops below 5.4 to 5.5. The drop in pH necessary for demineralization for cementum and dentin is 6.2 to 6.7.16 While results from studies on the effects of whitening on enamel surface morphology vary, it is generally accepted that whitening does affect tooth mineral content. Numerous techniques including FT-raman spectroscopy, atomic absorption spectroscopy (AAS), micro-computed tomography, inductively coupled plasma atomic emission spectroscopy (ICP-AES), ion chromatography (IC), and electron probe micro analysis (EPMA) have been used to evaluate changes in human enamel surface chemical composition (Figure 3). Enamel exposed to 10% carbamide peroxide solution for 6 hours lost an average of 1.06 ?g/mm2 of calcium as determined by AAS.8 For comparison, enamel that was also exposed to a cola beverage for 2.5 minutes, which is the approximate time teeth are exposed when drinking a 16-oz soft drink, experienced an average calcium loss of 1.25 ?g/mm2.8 These data provide a reference point for comparison of the potential demineralization effects from a daily whitening regimen (Figure 4). An EPMA of human enamel samples exposed to 10% carbamide peroxide for 336 hours, changed every 8 hours, showed lowered concentrations of calcium (C) and phosphorus (P) as well a decrease in Ca:P ratio.9 In a study employing ICP-AES and IC to measure mineral loss from bovine enamel by a 30% HP solution showed a decrease in mineral elements as well as a decrease in Ca:P ratio in whitened enamel.10
Although most studies reported a change in mineral content, the main conclusion drawn was that the measured loss was not clinically significant and that the whitening process did not pose a threat to enamel in the presence of human saliva.
MICROHARDNESS
Surface microhardness measurement is a relatively simple method to determine the ability of enamel and dentin to resist plastic deformation from a standard source. It is closely related to a loss or gain of mineral of the dental structure.19 Most studies have used either the Knoop hardness test or the Vickers test. In the Knoop hardness test, a pyramidal diamond is pressed onto the enamel specimen surface with a known force, for a specified time, and the resulting indentation is measured by a microscope.

A review of studies that used microhardness testing to evaluate structural enamel defects after whitening showed great inconsistency in the outcomes, most likely due to different study methodologies. The review showed that studies that simulated intraoral conditions closely by using human saliva and fluoride demonstrated a lower risk of enamel microhardness.22
REMINERALIZATION STRATEGIES
Demineralization associated with tooth whitening has been reviewed by looking at the enamel surface morphology, mineral loss, and microhardness. The majority of studies that closely replicated the dynamic intraoral environment showed that HP- and carbamide peroxide-containing products had no clinically significant adverse effects on enamel. However, two clinical cases did show significant enamel damage associated with the use of over-the-counter whitening products.23,24 Eventually, upcoming in situ and in vivo studies will provide more evidence on this topic.
In order to address the possible concerns of demineralization associated with whitening, several remineralization strategies using fluoride and calcium phosphate technologies have been suggested. Remineralization is the process whereby calcium and phosphate ions are supplied from a source externally to the tooth to promote ion deposition into the crystal voids in demineralized enamel to produce mineral gain.25 Calcium and phosphate ions are available in the human saliva but the net remineralization may be too small and slow.
Manufacturers have included fluoride, potassium nitrate, amorphous calcium phosphate (ACP), casein phosphopeptide-ACP, calcium sodium phosphosilicate, and tricalcium phosphate to promote remineralization, as well as reduce tooth sensitivity after whitening. Whether these active components truly assist in remineralization after whitening has yet to be determined. However, the application of topical fluoride postwhitening has been demonstrated to be effective in restoring the mineral content and microhardness values back to the baseline level.26,27
CONCLUSION
Tooth whitening is intended for improving tooth color and has become a widely accepted dental procedure in esthetic dentistry. Almost every procedure, including tooth brushing and oral prophylaxis, has some effect on the enamel. Based on the current literature, it seems that tooth whitening does not negatively effect enamel when conducted with proper dental supervision and appropriate selection of a neutral whitening agent.
REFERENCES
- Kwon S, Wertz PW, Li Y, Chan DC. Penetration patterns of rhodamine dyes into enamel and dentin: confocal laser microscopy observation. Int J Cosmet Sci. 2012;34:97–101.
- Albers H. Lightening natural teeth. ADEPT Report. 1991;2:1–24.
- Haywood VB. History, safety, and effectiveness of current bleaching techniques and applications of the nightguard vital bleaching technique. Quintessence Int. 1992;23:471–488.
- Li Y. Safety controversies in tooth bleaching. Dent Clin of North Am. 2011;55:255–263.
- Shannon H, Spencer P, Gross K, Tira D. Characterization of enamel exposed to 10% carbamide peroxide bleaching agents. Quintessence Int. 1993;24:39–44.
- Ben-Amar A, Liberman R, Gorfil C, Bernstein Y. Effect of mouthguard bleaching on enamel surface. Am J Dent. 1995;8:29–32.
- Yeh ST, Su Y, Lu YC, Lee SY. Surface changes and acid dissolution of enamel after carbamide peroxide bleach treatment. Oper Dent. 2005;30:507–515.
- McCracken MS, Haywood VB. Demineralization effects of 10 percent carbamide peroxide. J Dent. 1996;24:395–398.
- Potocnik I, Kosec L, Gaspersic D. Effect of 10% carbamide peroxide bleaching gel on enamel microhardness, microstructure, and mineral content. J Endod. 2000;26:203–206.
- Lee KH, Kim HI, Kim KH, Kwon YH. Mineral loss from bovine enamel by a 30% hydrogen peroxide solution. J Oral Rehabil. 2006;33:229–233.
- Al-Salehi SK, Wood DJ, Hatton PV. The effect of 24h non-stop hydrogen peroxide concentration on bovine enamel and dentine mineral content and microhardness. J Dent. 2007;35:845–850.
- Berger SB, Cavalli V, Martin AA, et al. Effects of combined use of light irradiation and 35% hydrogen peroxide for dental bleaching onhuman enamel mineral content. Photomed Laser Surg. 2010;28:533–538.
- Li Q, Xu BT, Li R, Yu H, Wang YN. Quantitative evaluation of colour regression and mineral content change of bleached teeth. J Dent. 2010;38:253–260.
- Lewinstein I, Fuhrer N, Churaru N, Cardash H. Effect of different peroxide bleaching regimens and subsequent fluoridation on the hardness of human enamel and dentin. J Prosthet Dent. 2004;92:337–342.
- Basting RT, Rodrigues AL Jr, Serra MC. The effect of 10% carbamide peroxide, carbopol and/or glycerin on enamel and dentin microhardness. Oper Dent. 2005;30:608–616.
- Peters MC. Strategies for noninvasive demineralized tissue repair. Dent Clin North Am. 2010;54:507–525.
- Scherer W, Penugonda B, Styner D, Georgescu M. At-home vital bleaching: effects on stained enamel and dentin. Pract Periodontics Aesthet Dent. 1992;4:11–15.
- Götz H, Duschner H, White DJ, Klukowska MA. Effects of elevated hydrogen peroxide “strip” bleaching on surface and subsurface enamel including subsurface histomorphology, micro-chemical composition and fluorescence changes. J Dent. 2007;35:457–466.
- Joiner A. Review of the effects of peroxide on enamel and dentine properties. J Dent. 2007;35:889–896.
- Sun L, Liang S, Sa Y, et al. Surface alteration of human tooth enamel subjected to acidic and neutral 30% hydrogen peroxide. J Dent. 2011;39:686–692.
- Nanci, A. Ten Cate’s Oral Histology. St. Louis: Mosby/Elsevier; 2008.
- Attin T, Schmidlin PR, Wegehaupt F, Wiegand A. Influence of study design on the impact of bleaching agents on dental enamel microhardness: a review. Dent Mater. 2009;25:143–157.
- Cubbon T, Ore D. Hard tissue and home tooth whiteners. CDS Rev. 1991;84:32–35.
- Hammel S. Do-it-yourself tooth whitening is risky. US News and World Report. 1998;66.
- Cochrane NJ, Cai F, Huq NL, Burrow MF, Reynolds EC. New approaches to enhanced remineralization of tooth enamel. J Dent Res. 2010;89:1187–1197.
- Bizhang M, Seemann R, Duve G, et al. Demineralization effects of 2 bleaching procedures on enamel surfaces with and without post-treatment fluoride application. Oper Dent. 2006;31:705–709.
- da Costa JB, Mazur RF. Effects of new formulas of bleaching gel and fluoride application on enamel microhardness: an in vitro study. Oper Dent. 2007;32:589–594.
From Dimensions of Dental Hygiene. May 2012; 10(5): 30, 32, 34, 39.

