
Bleeding on Probing Defined
A key element in periodontal risk assessment, bleeding on probing is most accurate when used in conjunction with other disease indicators.
This course was published in the May 2012 issue and expires May 2015. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the role of bleeding on probing (BOP) in periodontal disease risk assessment.
- Explain the efficacy of BOP as a periodontal disease indicator.
- Identify different systems of assessing gingival health.
- Detail the effects of dental biofilm on BOP.
INTRODUCTION
As our careers unfold and our experience in dental practice grows, it is important to regularly re-examine the basics and make sure our clinical practice is evidence-based and current. Probing periodontal pockets and recording the subsequent bleeding (or lack thereof) is one of the mainstays of periodontal management. This article, “Bleeding on Probing Defined,” will help ensure that the conclusions made from clinical bleeding on probing data are based on up-to-date scientific knowledge. The Colgate-Palmolive Company is delighted to have provided an unrestricted educational grant to support the second article of this four-part series “Partnering to Improve Periodontal Health” in collaboration with the American Academy of Periodontology.
—Barbara Shearer, BDS, MDS, PhD, Director of Scientific Affairs, Colgate Oral Pharmaceuticals
Traditionally, bleeding on probing (BOP) has been used to diagnose the presence of periodontal diseases, and it is a reliable indicator of gingival inflammation, especially when used in conjunction with other factors. Many believe that BOP also serves as an indicator of future clinical attachment loss, however, the evidence supporting this correlation is weak.1
BOP can be effective in the diagnosis and monitoring of active periodontal diseases,2,3 but when used as a stand-alone test, it can be inaccurate.3 BOP is not a definitive sign of periodontal diseases, as there are other factors besides gingival inflammation that can cause capillary fragility, and a comprehensive patient evaluation must be performed. A proper diagnosis should include a combination of BOP, probing depth, and clinical attachment loss. Assessments must include an evaluation of medical history, medication usage, physical disabilities, and systemic factors. Full-mouth radiographs can help clinicians estimate the amount of remaining alveolar bone, as well as provide valuable reference points for future assessments.
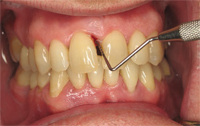
BOP is useful for predicting the progression of periodontitis, and pockets that bleed on two or three consecutive appointments are likely to become active.4 Sites that bleed on probing tend to have significantly more inflammation than nonbleeding sites.5 But the information collected during probing can be affected by a number of variables, including choice of probe, probing reproducibility, probe angulation, force used, site location, and gingival health status.
PROBING EFFICACY
Information collected through periodontal probing can be used to monitor pocket depth, bone loss, and attachment loss. It is useful in providing either positive predictive values or negative predictive values that illustrate disease progression. Predictive values are measurements used to interpret diagnostic test results.
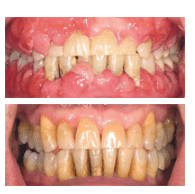
remove biofilm. Figure 2A (top) shows a patient before receiving scaling
and root planing. Figure 2B (bottom) is the patient 13 months later, after
receiving regular periodontal treatment, including scaling and root planing
and osseous surgery.
A positive predictive value indicates the percentage of people who both exhibit BOP and are correctly diagnosed with progressive periodontal diseases. A negative predictive value indicates the percentage of people whose BOP test results show no bleeding and no active disease progression. These values do not diagnose periodontal disease, but they do indicate a patient’s likelihood of developing disease in the future.
Clinical studies support the relevance of BOP in predicting the course of oral periodontal diseases,6 and they show the absence of BOP to be a reliable indicator of periodontal stability.
ASSESSING BLEEDING ON PROBING
Most clinicians use calibrated periodontal probes to assess BOP, but wooden interdental cleaners and dental floss are also utilized. Calibrated periodontal probes measure probing depth or the distance from the gingival margin to the base of the probeable crevice, and the clinical attachment loss—the distance from the cemento-enamel junction to the base of the probeable crevice (Figure 1). These measurements provide an approximation of periodontal pocket depth and are invaluable as reference points for monitoring disease progression.
Periodontal pockets are clinically important because they provide a habitat for periodontal pathogens. Deep pockets can be difficult for both the clinician and patient to clean, and they are more likely to harbor harmful periodontal pathogenic bacteria.
There are several gingival assessment systems used to quantify BOP. Each method can produce different results. Most gingival bleeding occurs during or immediately after probing, but some indices incorporate a time factor to allow for bleeding to begin.7 Other indices require the amount of gingival bleeding to be assessed.8 BOP may also increase if the patient has performed oral hygiene procedures just before being examined.9 Table 1 provides a description of the most popular gingival assessment systems.8, 10–13
VARIABLES THAT AFFECT BLEEDING ON PROBING
Bleeding can be more prevalent if a site is continually probed, but one of the most important variables is the force used when probing. If excessive force or incorrect technique is used, even healthy tissue may begin bleeding.14 Bleeding is also more likely to occur if the gingival tissue is thinner than normal.15
The accuracy of probing measurements also depends on the force used, the shape and size of the probe tip, and the level of inflammation in the tissues. In clinically healthy sites, the tissues are more toned and tend to have a “hammock” effect, so when a gentle insertion force is used, a probe is less likely to penetrate to the apical termination of the junctional epithelium. Studies have shown that when a force greater than 25 g is applied, bleeding at healthy sites can be induced.16
When untreated, diseased sites are probed, the probe tends to penetrate more deeply, which leads to overestimated probing measurements. On the other hand, post-treatment measurements tend to be underestimated, although the discrepancy is usually no more than a millimeter. Studies have shown that the probe with a diameter of 0.63 mm produces the most accurate results,17 as smaller probes often penetrate beyond the base of the pocket into the inflamed connective tissue. In heavily inflamed tissues, a probe can penetrate up to 1 mm into the connective tissue attachment.18
Higher probing forces are thought to traumatize tissues, cause bleeding, and lead to a false assumption of inflammation.19 Although higher probing forces lead to more reproducible readings, the use of lighter forces makes it easier to detect smaller changes in attachment levels. Clinicians tend to use greater force in posterior segments than in anterior segments.
PROBING REPRODUCIBILITY
One of the most important factors for collecting accurate results is probing reproducibility. A study that compared two probes set at 0.75 N found no differences in reproducibility,20 while another study looked at intra-examiner and inter-examiner reproducibility for threshold probing depths of greater than 1 mm and found an accuracy rate of 91.3%.21 The introduction of controlled-force probes has decreased much of the reproducibility error, but mistakes in manufacturing can still affect measurements. Tests have shown that the accuracy of probe markings can vary considerably from the manufacturer’s designated calibration.22 Probing depths can be affected by whether a tapered probe or a parallel-sided probe is used, with the parallel probe tending to result in deeper probing depths.23 However, when both types were compared, 89% of the results showed no difference.
THE ROLE OF BIOFILM IN BLEEDING ON PROBING
Gingivitis is caused by an overgrowth of indigenous microflora, which is both Gram negative and Gram positive. As the condition develops, the prevalence of Gram-negative Actinomyces species increases. Three species of Gram-negative and anaerobic or facultative bacteria are the main etiologic agents responsible for periodontal diseases.24 Bacteroides forsythus, Porphyromonas gingivalis, and Treponema denticola are most prevalent in clinical measures of periodontal diseases. About 25% of plaque will become Gram negative.
The process begins with glycoproteins from saliva binding to the surface of the enamel forming the pellicle. The first bacteria to attach to the pellicle are Gram-positive aerobic bacteria. After a few days, anaerobic Gram-negative species begin to colonize the biofilm, inducing an inflammatory response, while the biofilm itself is a constantly renewing source of lipopolysaccharides (LPS).Bacterial LPS are one of the major components of Gram-negative bacteria surface membranes, and they promote a strong immune response. Among individuals at risk of periodontitis, the biofilm will enter the gingival sulcus, disrupting the union between the coronal portion of the junctional epithelium (JE) and the tooth. As the JE is converted into pocket epithelium, a shallow gingival pocket is created that allows greater access for substances, such as bacterial LPS, to blood vessels and the connective tissues. Subgingival plaque is difficult to remove effectively through brushing, flossing, or mouthrinses. The most effective way to remove biofilm is through periodontal debridement (Figure 2A and Figure 2B).
RISK FACTORS THAT AFFECT PERIODONTAL THERAPY
Gingival bleeding can also be symptomatic of systemic issues, and unless these are treated correctly, periodontal therapy may not be sufficient to restore oral health. Systemic diseases, the use of over-the-counter or prescribed medications, and smoking can all affect periodontal health.
Smokers may exhibit less BOP because nicotine can suppress bleeding, causing vasoconstriction in peripheral blood vessels that can lead to a compromised immune response. An initial study showed a significant correlation between tobacco usage and gingivitis.25 In 1986, a study introduced experimental gingivitis to a group of dental students, half of whom were smokers.26 Although plaque formation rates were similar for both groups, the smokers exhibited a less pronounced inflammatory response. These findings were backed up in a study in 1990,27 which supports the theory that smokers have a reduced capacity to mount a defense against biofilm.
The presence of periodontitis can make it more difficult to control diabetes, and people with diabetes are more susceptible to periodontitis. Patients with diabetes undergoing periodontal therapy may end up needing less insulin,28 however, this isn’t always certain. The need for insulin may fluctuate because many patients start taking a greater interest in their health after undergoing periodontal treatment, and thus may become more compliant with their diabetes management regimen.
Patients taking anticoagulant agents, such as warfarin or heparin, that retard clotting may sometimes experience increased gingival bleeding. Individuals taking antiplatelet drugs following cardiac surgery are also at increased risk of prolonged and spontaneous gingival bleeding. Patients taking aspirin and nonsteroidal antiinflammatory drugs may experience increased blood loss if they are taken prior to periodontal surgery. Ibuprofen has been found to significantly increase intraoperative blood loss.29
TAKING ACTION
BOP is just one piece of the periodontal disease diagnosis puzzle. When BOP is the only symptom exhibited, getting patients to view their oral health as at risk can be difficult. Many patients feel that if they are not in pain, then nothing is wrong. Dental hygienists are well-suited to explain the role that BOP plays in patients’ oral health and encourage them to take action. Dental hygienists must not only convince patients of the existence and seriousness of their condition, but they need to also demonstrate how patients’ oral health will be affected in the future if appropriate action isn’t taken.
Table 1. Gingival Assessment System
Gingival Index
One of the most popular systems, the Gingival Index—introduced in 1963 by Loe and Silness—is based on inserting the probe apically to the gingival margin.10 It is generally used to assess the severity of gingivitis based on bleeding on probing (BOP). The tooth is examined on the lingual, mesial, distal, and buccal surfaces, and probed to test the degree of firmness. Numbers are used to evaluate the degree of inflammation, with a value of 0 given to normal gingiva; 1 for mild inflammation but no BOP; 2 for moderate inflammation and BOP; and 3 for gingiva exhibiting severe inflammation with a tendency to bleed spontaneously.
Eastman Interdental Bleeding Index
The Eastman Interdental Bleeding Index determines inflammation levels based on bleeding that occurs within 15 seconds after probing with a wooden interdental cleaner.
Papilla Bleeding Index
The Papilla Bleeding Index is based on sweeping a probe in the sulcus from the line angle to the interproximal contact.12
Papillary Bleeding Score
In 1979, the Papillary Bleeding Score was introduced by Loesche.8 It uses a triangular-shaped wooden toothpick to stimulate the interproximal gingival tissue—inspecting one quadrant at a time. A value of 0 is given to healthy gingival tissue; 1 for red tissue with no bleeding; 2 for bleeding without flow along the gingival margin; 3 for bleeding with flow along the gingival margin; 4 for copious amounts of bleeding; and 5 for severely inflamed tissue that has a tendency to spontaneously bleed.
Plaque Index
The Plaque Index was developed by Silness and Loe to assess the thickness of plaque at the cervical margin. This index requires that each tooth be dried and examined with an explorer passed over the distal, mesial, lingual, and buccal surfaces. Each surface is given a score between 0 and 3. A 0 indicates no plaque; 1 indicates nonvisible plaque that can only be detected by scraping with a probe; 2 indicates visible plaque; and 3 indicates abundant plaque. The scores for the individual surfaces of each tooth are then added up and divided by the number of sites assessed. Increased plaque build-up leads to increased BOP.
Ramfjord Teeth
Ramfjord Teeth (numbers 3, 9, 12, 19, 25, and 28) are used to assess the condition of the whole mouth. This type of partial-mouth assessment is an acceptable alternative to full-mouth examinations for epidemiologic studies of gingivitis, but is inadequate for epidemiologic studies of periodontitis.13
REFERENCES
- Badersten A, Nilvéus R, Egelberg J. Scores of plaque, bleeding, suppuration and probing depth to predict probing attachment loss. 5 years of observation following nonsurgical periodontal therapy. J Clin Periodontol. 1990;17:102–107.
- Claffey N, Engelberg J. Clinical indicators of probing attachment loss following initial periodontal treatment in advanced periodontitis patients. J Clin Periodontol. 1995;22:690–696.
- Haffajee AD, Socransky SS, Lindhe J, Kent RL, Okamoto H,Yoneyama T. Clinical risk indicators for periodontal attachment loss. J Clin Periodontol. 1991;18:117–125.
- Lindhe J, Haffajee AD, Socransky SS. Progression of periodontal disease in adult subjects in the absence of periodontal therapy. J Clin Periodontol. 1983;10:433–442.
- Greenstein G, Caton J, Polson AM. Histologic characteristics associated with bleeding after probing and visual signs of inflammation. J Periodontol. 1981;52:420–425.
- Lang NP, Adler R, Joss A, Nyman S. Absence of BOP. An indicator of periodontal stability. J Clin Periodontol. 1990;17:714–721.
- Caton JG, Polson AM. The interdental bleding index: a simplified procedure for monitoring gingival health. Compend Contin Educ Dent. 1985;6:88–92.
- Loesche WJ. Clinical and microbiological aspects of chemotherapeutic agents used according to the specific plaque hypothesis. J Dent Res. 1979;58:2404–2412.
- Abbas F, Voss S, Nijboer A, Hart AA, Van der Velden U. The effect of mechanical oral hygiene procedures on BOP. J Clin Periodontol.1990; 17:199–203.
- Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontal Scand. 1963;21:533–551.
- Muhlemann HR, Son S. Gingival sulcus bleeding—a leading symptom in initial gingivitis. Helv Odontol Acta. 1971;15:107–113.
- Caton J, Polson A, Bouwsma O, Blieden T, Frantz B, Espeland M.Associations between bleeding and visual signs of interdental gingival inflammation. J Periodontol. 1988;59:722–727.
- Fleiss JL, Park MH, Chilton NW, Alman JE, Feldman RS,Chauncey HH. Representativeness of the “Ramfjord teeth” for epidemiologic studies of gingivitis and periodontitis. Community Dent Oral Epidemiol. 1987;15:221–224.
- Karayiannis A, Lang NP, Joss A, Nyman S. BOP as it relates to probing pressure and gingival health with a reduced but healthy periodontium. A clinical study. J Clin Periodontol. 1992;19:471–475.
- Müller HP, Könönen E. Variance components of gingival thickness.J Periodontal Res. 2005;40:239–244.
- Lang NP, Nyman S, Senn C, Joss A. BOP as it relates to probing pressure and gingival health. J Clin Periodontol. 1991;18:257–261.
- Hassan MA, Bogle G, Quishenbery W, Stephens D, Riggs M,Egelberg J. Pain experienced by patients during periodontal recall examination using thinner versus thicker probes. J Periodontol. 2005;76:980–984.
- Listgarten MA. Periodontal probing: what does it mean? J Clin Periodontol. 1980;7:165–176.
- Mombelli A, Mühle T, Frigg R. Depth-force patterns of periodontal probing. Attachment-gain in relation to probing force.J Clin Periodontol. 1992;19:295–300.
- van der Velden U, de Vries JH. The influence of probing force on the reproducibility of pocket depth measurements. J Clin Periodontol.1980;7:414–420.
- Aeppli D, Boen JR, Bandt CL. Measuring and interpreting increases in probing depth and attachment loss. J Periodontol.1985;56:262–264.
- Van der Zee E, Davies EH, Newman HN. Marking width, calibration from tip and tine diameter of periodontal probes. J Clin Periodontol. 1991;18:516–520.
- Atassi F, Newman HN, Bulman JS. Probe tine diameter and probing depth. J Clin Periodontol. 1992;19:301–304.
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr.Microbial complexes in subgingival plaque. J Clin Periodontol.1998;25:134–144
- Arno A, Waerhaug J, Lovdal A, Schei O. Incidence of gingivitis as related to sex, occupation, tobacco consumption, toothbrushing,and age. Oral Surg Oral Med Oral Pathol. 1958;11:587–595.
- Bergström J, Preber H. The influence of cigarette smoking on the development of experimental gingivitis. J Periodontal Res.1986;21:668–676.
- Danielsen B, Manji F, Nagelkerke N, Fejerskov O, Baelum V.Effect of cigarette smoking on the transition dynamics in experimental gingivitis. J Clin Periodontol. 1990;17:159–164.
- Williams RC Jr, Mahan CJ. Periodontal disease and diabetes inyoung adults. JAMA. 1960;172:776–778.
- Braganza A, Bissada N, Hatch C, Ficara A. The effect of nonsteroidalanti-inflammatory drugs on bleeding during periodontal surgery. J Periodontol. 2005;76:1154–1160.
From Dimensions of Dental Hygiene. May 2012; 10(5): 23-26.



