 JIM DOWDALLS/SCIENCE SOURCE
JIM DOWDALLS/SCIENCE SOURCE
The Bidirectional Link
Dental professionals should be well-versed in the relationship between periodontal diseases and diabetes so they can better serve patients with this common metabolic condition.
This course was published in the March 2014 issue and expires March 31, 2017. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss how the presence of advanced glycation end products, apoptosis, and impaired wound healing among people with diabetes affects their risk of periodontal diseases.
- Identify the negative effects that periodontal diseases exert on insulin resistance and kidney health.
- List the treatment options available for patients with diabetes.
- Explain the effects of periodontal treatment on diabetes management.
In this continuing education piece sponsored by Colgate and created in collaboration with the American Academy of Periodontology, Vanchit John, DDS, MSD, and Tatiana de Bedout provide an in-depth look at the intricate relationship between periodontitis and diabetes.
Introduction
The Colgate-Palmolive Company supported a joint workshop of the American Academy of Periodontology (AAP) and the European Federation of Periodontology (EFP) on periodontitis and systemic diseases, which was held in Segovia, Spain, in November 2012. More than 90 experts attended the workshop and reviewed the evidence relating to the association between periodontal diseases and diabetes mellitus, cardiovascular disease, adverse pregnancy outcomes, and other systemic diseases. At the heart of this workshop was the desire to provide practicing oral health professionals with evidence-based information and recommendations for day-to-day patient management. The proceedings were published in April 2013 in a joint publication of the AAP and EFP.
Colgate is committed to ensuring that practicing dental professionals have access to the latest educational resources and is delighted to have provided an unrestricted educational grant to support this article, “The Bidirectional Link,” in collaboration with the AAP. The piece provides a useful review of the literature, highlighting the mechanisms behind the bidirectional relationships between periodontal diseases and diabetes. As an oral health professional, you have a unique opportunity to improve patient outcomes with periodontal disease management and patient education.
I hope you find this article a valuable resource in the management of patients with diabetes in your practice.
——Barbara Shearer, BDS, MDS, PhD
Director of Scientific Affairs
Colgate Oral Pharmaceuticals
Diabetes mellitus is a metabolic disease characterized by the loss of control of glucose homeostasis, along with changes affecting fat and protein metabolism. This loss of control may be due to a defect
in insulin secretion, insulin action, or both. Diabetes is a chronic disease that is a public health problem of global significance. Approximately 347 million adults worldwide have type 2 diabetes.1 In the United States, approximately 25.8 million people, or roughly 8.3% of the population, are affected.2 Of this group, approximately 18.8 million people have been diagnosed with diabetes and about 7 million people may be undiagnosed. Diabetes is a leading cause of kidney failure, nontraumatic lower-limb amputations, and blindness among adults in the US. Diabetes is also a major cause of heart disease and stroke, and is the seventh leading cause of death in this country.2
Periodontal diseases are common chronic inflammatory conditions. According to a 2012 survey, the prevalence of periodontal diseases in the US population is approximately 64.7 million adults.2 Data showed that of those affected, 8.7% had mild chronic periodontitis (Figure 1), 30% presented with moderate chronic periodontitis, and 8.5% had severe chronic periodontitis (Figure 2). In adults age 65 and older, 64% had either moderate or severe chronic periodontitis.2 Clearly, periodontitis is a significant health problem in the US, with disparities existing among different sociodemographic segments.
Oral complications abound in those with uncontrolled diabetes. They may include: xerostomia; propensity for infection; poor wound healing; and increased incidence of caries, gingivitis, and periodontitis. Consequently, oral health professionals need to remain up to date on the association between periodontal diseases and diabetes. The available research on the subject is robust, with more than 200 articles published that explore this relationship.3
Approximately one-third of people with diabetes have severe periodontal disease with attachment loss measuring 5 mm or greater.4 The relationship between diabetes and periodontal diseases may be due to microvascular changes,5 changes in the composition of the gingival crevicular fluid,6 alteration in collagen metabolism,7 changes in the host response,8 mutations in the subgingival microflora,6 genetic factors,9 and nonenzymatic glycation leading to an accumulation of advanced glycation end products (AGEs).10 The role of increased apoptosis (programmed cell death) on wound healing and bone loss may also be related.10,11
ADVANCED GLYCATION END PRODUCTS
AGEs are referred to as “long-lived molecules formed by the irreversible binding of glucose to protein and lipids in plasma, as well as the tissues during persisting hyperglycemia.”12 These molecules form under normal conditions and accumulate during aging. However, this process is enhanced during hyperglycemic conditions found in diabetes.12 AGEs interact with cell receptors, such as the receptor for advanced glycation end products (RAGE), which is a member of the immunoglobulin superfamily of cell surface molecules. The AGE-RAGE interaction leads to the initiation of intracellular signaling pathways—which is partly mediated by enhanced cellular oxidant stress—thus encouraging the creation of a pro-inflammatory environment.13 The resulting inflammatory activity can lead to vascular alterations, development of vascular lesions, and impairment of normal reparative responses, all of which impede the healing process. In addition, AGEs affect wound healing by stimulating apoptosis and affecting the remodeling of the extracellular matrices.14 Alterations in bone metabolism and interferences with osteoblast differentiation are also possible.15
APOPTOSIS
 Apoptosis is the process of programmed cell death that occurs in multicellular organisms. AGEs can affect both connective tissue and bone by promoting the apoptosis of matrix-producing cells. This programmed cell death can affect wound healing, which is hindered significantly in patients with uncontrolled diabetes. Apoptosis of matrix-producing cells during the healing process can interfere with the production of cellular matrix, thus limiting repair.16 Diabetes may also increase the death of cells that line the bone, such as osteoblasts.17 Uncoupling of the healthy resorption and formation cycle leads to a net loss of bone. He et al17 suggested that the periodontal bone loss in patients with diabetes may be due to the reduction in matrix formation caused by increased apoptosis of the matrix-forming cells.
Apoptosis is the process of programmed cell death that occurs in multicellular organisms. AGEs can affect both connective tissue and bone by promoting the apoptosis of matrix-producing cells. This programmed cell death can affect wound healing, which is hindered significantly in patients with uncontrolled diabetes. Apoptosis of matrix-producing cells during the healing process can interfere with the production of cellular matrix, thus limiting repair.16 Diabetes may also increase the death of cells that line the bone, such as osteoblasts.17 Uncoupling of the healthy resorption and formation cycle leads to a net loss of bone. He et al17 suggested that the periodontal bone loss in patients with diabetes may be due to the reduction in matrix formation caused by increased apoptosis of the matrix-forming cells.
In cell culture studies, monocytes from patients with diabetes, when stimulated with lipopolysaccharides found in the outer membrane of Gram-negative bacteria, produced greater amounts of tumor necrosis factor-alpha (TNF-alpha) compared to those without diabetes.18 TNF-alpha, an adipokine involved in systemic inflammation, is a potent inducer of apoptosis in fibroblasts and osteoblasts. Taylor et al12 noted that diabetes enhanced the inflammatory response to oral bacteria associated with periodontal diseases. The increase in inflammation affected bone levels via increased bone resorption while inhibiting bone formation. This associated resorption is mediated through a reduction in the number of osteoblasts as a result of the effects of inflammation on apoptosis and the proliferation and/or the differentiation of bone-lining cells (Figure 3).
WOUND HEALING
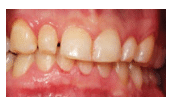
FIGURE 1 COURTESY OF SAYIJ MAKKATIL, BDS
Wound healing is impaired in patients with diabetes. During the inflammatory phase of wound healing, the monocyte/macrophage interaction is key.19 This interaction is significant in signaling and transitioning from the inflammatory to the granulation phase of the healing process. Hyperlipidemia, often seen in patients with diabetes, affects the function of monocyte cell membrane-bound receptors and enzyme systems, leading to a slowing down of the wound healing process.20 Furthermore, AGEs affect the differentiation and maturation of monocytes. This leads to an increase in the inflammatory response, with resultant elevation in tissue destruction (Figure 4).20
PERIODONTAL DISEASES
Periodontal diseases, which are chronic inflammatory and infectious conditions, can negatively impact glycemic control and other diabetes-associated systemic complications.21,22 Complications associated with diabetes and periodontal diseases may be preceded by a hyperactive innate immune response.21 Untreated periodontal diseases can result in changes to the soft and hard tissues of the periodontium, including ulcerations in the pocket epithelium. Systemic bacteremia can result, increasing the presence of Gram-negative and obligate anaerobe bacteria in the blood stream. Additionally, bacterial components, such as lipopolysaccharides, are also evident in the blood stream. The pro-inflammatory cytokines—interleukin (IL)-1 beta and TNF-alpha—are stimulated, along with IL-6 and prostaglandin E2. These cytokines can affect glucose and lipid metabolism, along with the actions of insulin.22
THE ROLE OF OBESITY
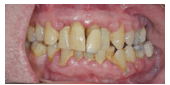
FIGURE 2 COURTESY OF YUSUKE HAMADA, BDS
Obesity is a risk factor for the development of diabetes.1 Adipose tissue, by virtue of secreting adipokines, can activate the innate immune system and serve as a mediator of a low-grade chronic systemic inflammation. This occurs through the production of pro-inflammatory cytokines, including TNF-alpha, IL-1beta, and IL-6—which are also produced in periodontal tissues during the periodontal disease process. Elevated levels of these cytokines affect the action of insulin and increase insulin resistance, which ultimately leads to hyperglycemia (Figure 5).23
INSULIN RESISTANCE AND RENAL DYSFUNCTION
Severe periodontal diseases can cause insulin resistance.24 This interaction is likely due to stimulation of hepatocytes, leading to secretion of acute phase proteins that include C-reactive proteins along with TNF-alpha. In muscle cells, TNF-alpha has been reported to cause insulin resistance.25
Research shows that patients who need renal dialysis develop more severe forms of periodontitis.26 Furthermore, periodontally involved patients with diabetes may also have microalbuminuria (when the kidney leaks small amounts of albumin into the urine), indicating a bidirectional association between periodontal diseases and kidney disease.27,28
OXIDATIVE STRESS AND MITOCHONDRIAL DYSFUNCTION
Oxidative stress refers to an imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects through neutralization by antioxidants. Recently, Bullon at al29 reviewed the role of oxidative stress and mitochondrial dysfunction as a common link between obesity, diabetes, atherosclerosis, and chronic periodontitis. Mitochondrial functions include generating cell energy, along with cell signaling, cell differentiation, cell-cycle control, and apoptosis. Metabolic and oxidative stress seen in these conditions can lead to an inflammatory response and cell organelle dysfunction,30 and may serve as the common link between obesity, diabetes, atherosclerosis, and chronic periodontitis.
TREATMENT OF TYPE 2 DIABETES
Studies show that maintaining healthy glycemic levels positively impacts diabetes-related complications, including retinopathy, nephropathy, and neuropathy.31–33 The American Diabetes Association recommends a glycosylated hemoglobin value (HbA1c) of 7% or less.34,35 This test measures the average levels of glycemia over a 2-month to 3-month period. It is, therefore, the most accurate way to detect how well patients are maintaining their blood sugar levels. The target fasting and preprandial levels of plasma or capillary glucose are between 70 mg/dL and 130 mg/dL.
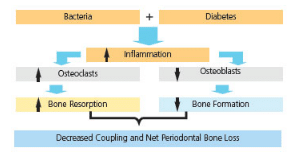
Reprinted with permission from: Genco RJ, Williams RC, eds. Periodontal Disease and Overall Health: A Clinician’s Guide. Yardley, Pa: Professional Audience Communications; 2010:90.
Pharmacologic management of diabetes includes the use of glucophage, sulfonylureas, glinides, alpha-glucosidase inhibitors, thiazolidinediones, and insulin. However, new treatment regimens have emerged, such as incretin-based therapies and amylin agonists.
Incretin hormones are involved in the regulation of blood glucose and, to a lesser extent, insulin and glucagon secretion.36,37 These hormones are released from endocrine cells in the small intestine in response to food. Activation of G protein-coupled receptors on pancreatic beta-cells leads to stimulation of insulin secretion. In type 2 diabetes, incretin function is impaired. Incretin-based therapies include the use of glucagon-like peptide-1 receptor agonists (GLP-1) and dipeptidyl peptidase-4 inhibitors (DPP-4). Therapeutic GLP-1 receptor agonists enhance insulin release and inhibit glucagon secretion. Additionally, development of DPP-4-resistant GLP-1 analogues, as well as agents that inhibit the enzymatic activity of DPP-4, help maintain incretin action.38,39
Amylin, a neuroendocrine hormone, is secreted along with insulin in response to food intake. This hormone causes inhibition of post-prandial glucagon secretion, slowing the rate of gastric emptying, enhancing satiety, and reducing food intake. In type 2 diabetes, amylin and insulin response is markedly impaired. Pramlintide, a synthetic analogue of the beta-cell hormone amylin, slows gastric emptying and inhibits glucagon production in a glucose-dependent fashion, producing A1c reductions of 0.5% to 0.7%.40
Other treatments include the use of colesevelam and bromocriptine, which have been approved by the US Food and Drug Administration for the treatment of type 2 diabetes. Colesevelam, which sequesters bile acid, has been used for the treatment of hyperlipidemia. It is thought to delay or alter absorption of glucose from the intestines. Bromocriptine, a dopamine-2 receptor agonist, has been shown to reduce A1c levels by approximately 0.6% as a monotherapy and 1.2% in combination with insulin or a sulfonylurea. The use of sodium-glucose transporter 2 blockers, which prevent renal glucose reabsorption and lower serum glucose by increasing urinary excretion of glucose, may also be effective.41
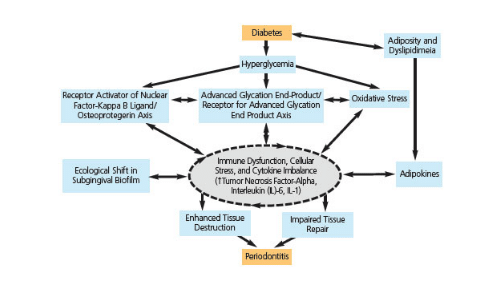
Reprinted with permission from: Taylor JJ, Preshaw PM, Lalla E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes. J Clin Periodontol. 2013;40(Suppl 14):129.
EFFECTS OF PERIODONTAL TREATMENT ON DIABETES
Engebretson and Kocher42 reported on two systematic reviews conducted in 2010. The studies included those that had at least a 3-month follow up on the effects of periodontal treatment on patients with type 2 diabetes. They concluded that a consistent, moderate improvement was noted on HbA1c levels in response to periodontal treatment. Taylor et al12 reviewed a heterogeneous set of 31 studies that included 10 randomized clinical trials (RCTs) and 21 nonRCTs. Six of the 10 RCTs reported a beneficial effect of periodontal treatment on glycemic control. Of the 21 nonRCTs, 12 studies reported a beneficial effect on glycemic control. Nine studies, however, did not report any improvement.
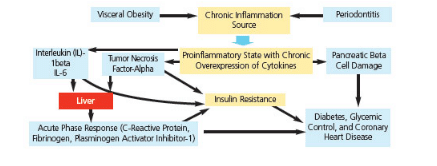
Adapted with permission from Donahue RP, Wu T. Ann Periodontol. 2001;6:119–124; reprinted from Genco RJ, Williams RC, eds. Periodontal Disease and Overall Health: A Clinician’s Guide. Yardley, Pa: Professional Audience Communications; 2010:94.
Recently, Engebretson et al43 reported on findings from a RCT that was stopped early because nonsurgical periodontal therapy did not improve glycemic control in patients with type 2 diabetes and moderate to advanced chronic periodontitis. Accordingly, the authors concluded that findings from this RCT did not support the use of nonsurgical periodontal treatment in patients with diabetes for the purpose of lowering levels of HbA1c. The American Academy of Periodontology (AAP) offered the following statement in response to Engebretson’s report: “As a number of population studies suggest, there is indeed a relationship between diabetes and periodontal disease. While this study specifically focuses on basic nonsurgical periodontal care, some cases of periodontal disease require more intensive treatment. There is evidence that more intensive periodontal therapies may be effective in glycemic control.”44
In the consensus report of the joint European Federation of Periodontology and the AAP, several guidelines were suggested to support health care professionals in their treatment of patients with diabetes. The guidelines recommended that patients with diabetes should receive the following:
- Explanation about the bidirectional relationship between periodontal diseases and diabetes
- Comprehensive oral exam that includes a complete periodontal exam
- Oral health education
- Annual oral screening for children and adolescents with diabetes
- Discussion of the increased risk for oral fungal infections and impaired wound healing45
SUMMARY
Diabetes and periodontitis are chronic disease conditions that have significant national and global implications. They also have a bidirectional relationship. Severe periodontitis affects HbA1c levels in patients with diabetes, who are also more likely to experience moderate to severe chronic periodontitis. Treatment of both diseases, along with patient education, will improve overall health. Research continues to be conducted on the relationship between these two chronic disease conditions. Health care professionals who treat patients with diabetes should remain up to date on advances in the understanding of diabetes, as well as guidelines and treatment recommendations.
REFERENCES
- Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40.
- Eke PI, Dye BA, Wei L, et al. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91:914‒920.
- Mealey BL, Ocampo GL. Diabetes mellitus and periodontal disease. Periodontol 2000. 2007:44:127‒153.
- US Department of Health and Human Services. National Diabetes Statistics, 2011. Available at: diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm. Accessed February 12, 2014.
- Verma S. C-reactive protein incites atherosclerosis. Can J Cardiol. 2004;20(Suppl B):29B‒31B.
- Kurtis B, Develioglu H, Taner IL, Balos K, Tekin IO. IL-6 levels in gingival crevicular fluid (GCF) from patients with non-insulin dependent diabetes mellitus (NIDDM), adult periodontitis and healthy subjects. J Oral Sci. 1999;41:163‒167.
- Willershauschen-Zonnchen B, Lemmen C, Hamm G. Influence of high glucose concentrations on glycosaminoglycan and collagen synthesis in cultured human gingival fibroblasts. J Clin Periodontol. 1991;18:190‒195.
- Sastrowijoto SH, Abbas F, Abraham-Inpijn L, van der Velden U. Relationship between bleeding/plaque ratio, family history of diabetes mellitus and impaired glucose tolerance. J Clin Periodontol. 1990;17:55‒60.
- Lalla E, Lamster IB, Feit M, et al. Blockade of RAGE suppresses periodontitis-associated bone loss in diabetic mice. J Clin Invest. 2000;105:1117‒1124.
- Liu R, Bal HS, Desta T, Behl Y, Graves DT. Tumor necrosis factor-alpha mediates diabetes-enhanced apoptosis of matrix producing cells and impairs diabetic healing. Am J Pathol. 2006;168:757‒764.
- Graves DT, Liu R, Oates TW. Diabetes-enhanced inflammation and apoptosis: impact on periodontal pathosis. Periodontol 2000. 2007;45:128‒137.
- Taylor GW, Borgnakke WS, Graves DT. Association between periodontal diseases and diabetes mellitus. In: Genco RJ, Williams RC, ed. Periodontal Disease and Overall Health; A Clinician’s Guide. Yardley, Pa: Professional Audience Communications; 2010: 83‒104.
- Lalla E, Lamster IB, Schmidt AM. Enhanced interaction of advanced glycation end products with their cellular receptor RAGE: implications for the pathogenesis of accelerated periodontal disease in diabetes. J Periodontol. 1998;3:13‒19.
- Vlassara H, Palace MR. Diabetes and advanced glycation end products. J Intern Med. 2002:251:87‒101.
- Santana RB, Xu L, Chase HB, Amar S, Graves DT, Trackman PC. A role for advanced glycation end products in diminished bone healing in type 1 diabetes. Diabetes. 2003;52:1502‒1510.
- Rai NK, Suryabhan, Ansari M, Kumar M, Shukla VK, Tripathi K. Effect of glycemic control on apoptosis in diabetic wounds. J Wound Care. 2005;14:277‒281.
- He H, Liu R, Desta T, Leone C, Gerstenfeld LC, Graves DT. Diabetes causes decreased osteoclastogenesis, reduced bone formation and enhanced apoptosis of osteoblastic cells in bacteria stimulated bone loss. Endocrinology. 2004:145:447‒452.
- Salvi GE, Collins JG, Yalda B, Arnold RR, Lang NP, Offenbacher S. Monocytic TNF alpha secretion patterns in IDDM patients with periodontal diseases. J Clin Periodontol. 1997:24:8‒16.
- Iacopino AM. Diabetic periodontitis: possible lipid-induced defect in tissue repair through alteration of macrophage phenotype and function. Oral Dis. 1995;1:214‒229.
- Cutler CW, Iacopino AM. Periodontal disease: links with serum lipid/triglyceride levels? Review and new data. J Int Acad Periodontol. 2003;5:47‒51.
- Grossi SG, Skrepcinski FB, DeCaro T, et al. Treatment of periodontal disease in diabetics reduces glycated hemoglobin. J Periodontol. 1997:68:713‒719.
- Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76(Suppl 11):2106‒2115.
- Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007:132:2169‒2180.
- Nishimura F, Murayama Y. Periodontal inflammation and insulin resistance—lessons from obesity. J Dent Res. 2001:80:1690‒1694.
- Ciaraldi TP, Carter L, Mudaliar S, Kern PA, Henry RR. Effects of tumor necrosis factor-alpha on glucose metabolism in cultured human muscle cells from nondiabetic and type 2 diabetic subjects. Endocrinology. 1998:139:4793‒4800.
- Naugle K, Darby ML, Bauman DB, Lineberger LT, Powers R. The oral health status of individuals with on renal dialysis. Ann Periodontol. 1998:3:197‒205.
- Kuroe A, Taniguchi A, Sekiguchi A, et al. Prevalence of periodontal bacterial infection in non-obese Japanese type 2 diabetic patients: relationship with C-reactive protein and albuminuria. Horm Metab Res. 2004:36:116‒118.
- Nishimura F, Iwamoto Y, Soga Y. The periodontal host response with diabetes. Periodontol 2000. 2007;43:245‒253.
- Bullon P, Newman HN, Battino M. Obesity, diabetes mellitus, atherosclerosis and chronic periodontitis: a shared pathology via oxidative stress and mitochondrial dysfunction? Periodontol 2000. 2014;64:139‒153.
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006:444:860‒867.
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977‒986.
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complication in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837‒853.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352:854‒865.
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193‒203.
- American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care. 2011;34(Suppl 1):S11‒61.
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696‒1705.
- Porte D, Sherwin RS, Baron A. Ellenberg & Rifkin’s Diabetes Mellitus. 6th ed. New York: McGraw-Hill; 2003.
- Triplitt CL. New technologies and therapies in the management of diabetes. Am J Manag Care. 2007;13(Suppl 2):S47‒54.
- Fonseca V. Expert Column: DPP-IV Inhibitors for the Treatment of Type 2 Diabetes. Available at: medscape.com/index/section_2783_0. Accessed February 13, 2014.
- Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29:1963‒1972.
- Nyenwe EA, Jerkins TW, Umpierrez GE, Kitabchi AE. Management of type 2 diabetes: evolving strategies for the treatment of patients with type 2 diabetes. Metabolism. 2011;60:1‒23.
- Engebretson S, Kocher T. Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. J Clin Periodontol. 2013;40(Suppl 14):S153‒163.
- Engebretson SP, Hyman LG, Michalowicz BS, et al. The effect of nonsurgical periodontal therapy on hemoglobin A1c levels in persons with type 2 diabetes and chronic periodontitis: a randomized clinical trial. JAMA. 2013;310:2523‒2532.
- American Academy of Periodontology. Basic care for periodontal disease may not enough for patients with diabetes. Available at: perio.org/consumer/Nonsurgical_Diabetes. Accessed February 12, 2014.
- Chapple IL, Genco R, working group 2 of the joint EFP/AAP workshop. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol. 2013;84(Suppl 4):S106‒112.
From Dimensions of Dental Hygiene. March 2014;12(3):55–60.



