GEVENDE/ISTOCK/GETTY IMAGES PLUS
GEVENDE/ISTOCK/GETTY IMAGES PLUS
The Role of Oral-Fluid Biomarkers in Diagnosing Oral Diseases
Dental hygienists are uniquely positioned to screen patients for systemic and oral diseases using salivary diagnostics.
This course was published in the April 2021 issue and expires April 2024. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Identify the primary salivary biomarkers associated with periodontal diseases, caries, and oral cancer.
- Explain the role of biomarkers in the pathogenesis of periodontal diseases.
- Describe how salivary testing has advanced the understanding and treatment of COVID-19.
- Discuss applications of point of care technology in a dental office.
Salivary diagnostics have been used to diagnose and monitor systemic disease since the late 1980s when highly sensitive tests were developed to detect hormones and drugs and monitor microbial infections. By the early 2000s, research was initiated to develop a comprehensive catalog of all salivary secretory proteins titled, “Human Salivary Proteome Project.” Salivary diagnostic testing is currently available for detection of illicit drugs, drug metabolism, and some infectious diseases such as human immunodeficiency virus (HIV), human papillomavirus (HPV), and Candida. Most recently, saliva has been examined as a biofluid to identify the presence of SARS-CoV-2, the virus that causes COVID-19. Diagnosing systemic disease is challenging and an invasive approach is often needed. However, salivary diagnostics is a minimally invasive approach to diagnosing and managing oral and systemic disease.1-4
Despite advances in knowledge and treatment of oral diseases, their burden remains high. In the United States, approximately 46% of adults older than 30 display signs of periodontal diseases, of which approximately 9% are severe. Furthermore, 26% of adults have untreated tooth decay with an even higher prevalence among certain minority populations. In 2019, approximately 53,000 new cases of oral and pharynx cancer were diagnosed in the US, which accounts for roughly 3% of all new cancer cases. As such, diagnosing disease early is critical for improved prognosis and treatment.4–6
ORAL FLUIDS
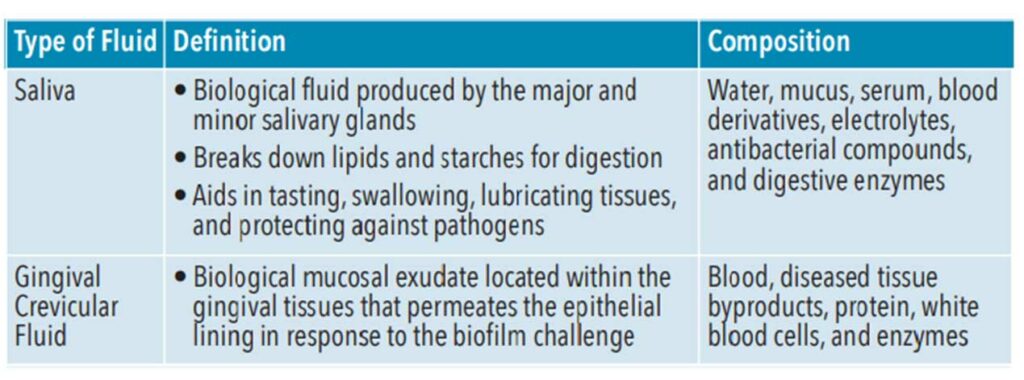
Saliva contains biomarkers, or biological substances that can be quantified and analyzed to indicate health and predict or detect disease. Saliva is a biological liquid composed of multiple components including water, mucus, serum, blood derivatives, electrolytes, antibacterial compounds, and digestive enzymes (Table 1).4,7,8 These include genome (DNA), proteome (protein), transcriptome (RNA), microRNA (miRNA), metabolome (metabolites), and microbiome (microorganisms). Saliva contains high-quality circulating cell-free DNA of which 70% originates from the host. The most recent advances in salivary diagnostics have been through the analysis of nucleic acids. Additionally, when compared to blood plasma levels, saliva contains more than 3,652 proteins and 12,562 peptides, which is almost 51% of the proteins and 79% of the peptides found in plasma.9-11
Gingival crevicular fluid (GCF) is a biological mucosal exudate located within the gingival tissues and is an indicator of periodontal health (Table 1). It is a complex serum composed of molecules originating from blood, diseased tissue byproducts, protein, white blood cells, and enzymes that permeate the epithelial lining of diseased soft tissues in response to the biofilm challenge. Biomarkers found in GCF—specifically interleukin (IL)-1β and IL-6—show promise as indicators of periodontal diseases.12,13 Used as a site-specific tool to detect and monitor periodontal health GCF’s complexity makes it a rich source of oral fluid biomarkers.
SALIVARY DIAGNOSTICS
Blood and saliva are similar as complex biofluids containing molecular proteins and peptides. Traditionally, blood or serum has been the preferred source for biomarkers to detect and monitor diseases and conditions. Collecting and analyzing blood samples can be costly, painful, and invasive, which makes salivary diagnostics advantageous. Blood draws must be collected by a trained healthcare professional, while saliva collection can be done by anyone or the patient him/herself. Painless compared to blood draws and tissue biopsies, salivary diagnostics poses less risk to the healthcare personnel handling the samples and reduces the risk of cross-contamination. For example, saliva contains factors that limit the infectivity of HIV, which makes it extremely low or insignificant risk for oral transmission. Additionally, blood draws can be problematic because blood begins to clot during the process. Salivary diagnostics is also less expensive. Sampling, shipping, and storage are all more economical, and saliva is readily available, which can reduce cost to patients and healthcare providers.7,14 Saliva is a promising and accurate predictor of early disease, improved diagnoses, and monitor of health.4 Diagnosing disease at earlier stages can be financially advantageous to the patient and lead to improved patient outcomes.
BIOMARKERS OF PERIODONTAL DISEASES
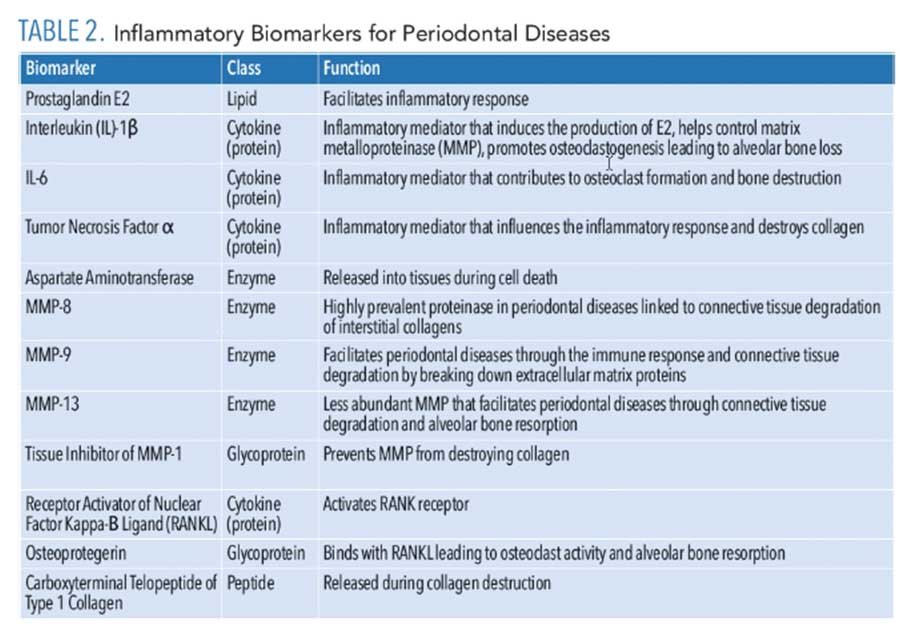
While initiation of periodontal diseases begins with bacterial pathogens, the host response causes the degradation of the connective tissue and bone. During the inflammatory response, many biomarkers are released that can be analyzed to detect and manage periodontal diseases (Table 2). An inflammatory response to the pathogenic bacteria within biofilm is initiated by the host, resulting in the release of lipopolysaccharides, cytokines, and chemokines such as prostaglandin E2, IL-1β, IL-6, and tumor necrosis factor-alpha (TNF-α).8,15,16 As periodontal disease advances from tissue inflammation to collagen-destroying enzymes, matrix metalloproteinase (MMPs) and aspartate aminotransferase are released from the cells. As periodontal disease worsens, TNF, IL-1β, and receptor activator of nuclear factor kappa-Β ligand (RANKL) elevate, leading to the breakdown of alveolar bone.8
IL-1β plays a significant role in chronic inflammation. Specifically, it is strongly associated with periodontal disease progression, and is considered a reputable biomarker in distinguishing active and inactive periodontal disease status.8 Increased levels of IL-1β can be detected in the GCF of individuals with chronic periodontal disease.16 Similar to IL-1β, IL-6 is a cytokine with an active role in the immune and inflammatory responses. Increased levels of IL-6 can be detected in the presence of gingivitis and periodontitis. IL-6 contributes to osteoclast formation and bone destruction in periodontal diseases. TNF-α is another contributor to the inflammatory and immune response. This biomarker is produced by macrophages and is a significant influencer in many systemic inflammatory responses. Like IL-1β, TNF-α can destroy collagen. TNF-α can be detected at higher levels in chronic periodontitis.8
As periodontal disease progresses, connective tissue is destroyed. MMP-8 is a predominant proteinase associated with periodontal diseases, leading to destroyed interstitial collagen. Monitoring of MMP-8 can determine periodontal status. Increased levels of MMP-8 are linked to increased bleeding on probing, clinical attachment loss, and periodontal pocket depth. Advanced periodontal disease leads to destruction of the alveolar bone. Osteoprotegerin, a glycoprotein that contributes to health of bone tissue, binds with RANKL, increasing osteoclast activity by releasing carboxy-terminal telopeptide of type I, collagen, leading to further bone resorption.8 Levels of osteoprotegerin can be an indicator of periodontal disease. Decreased levels of IL-1β, MMP-8, TNFα, and osteoprotegerin can be observed by completing nonsurgical periodontal therapy.8,16 The enzymes and proteins released are biomarkers that can be potentially isolated to aid in a periodontal disease diagnosis.
BIOMARKERS OF ORAL CANCER
While saliva has been examined as a medium to detect many types of cancer, it demonstrates the greatest potential to detect oral cancer because saliva is in direct contact with the cancerous lesion, and it sheds detectable cancerous cells.17 Prior to an oral cancer diagnosis, patients often have precancerous lesions such as leukoplakia, erythroplakia, or lichen planus.18 Tissue biopsy is the industry standard for diagnosing cancer; however, it is expensive, invasive, painful, and requires a trained professional to perform. Several biomarkers have been linked to an oral squamous cell carcinoma (OSCC) diagnosis, including IL-1β, IL-8, Mac‐2‐binding protein, profilin 1, catalase proteins, defensin-1, endothelin receptor type B hypermethylation, ribonucleic acids (RNA), and microRNAs.4,19 Biomarkers found in RNA have an 81% accuracy rate in the detection of OSCC. High levels of tumor markers have also been identified in the saliva of individuals with cancer. Tumor-specific DNA markers were found in the saliva of 100% of patients with oral cancer and between 47% and 70% of patients with tumors in other areas of the body. Additionally, cortisol levels in saliva show potential as a biomarker for OSCC due to detecting significantly higher levels in OSCC patients.4
BIOMARKERS OF CARIES
Biomarkers within saliva—such as glycoproteins, peptides, microbes, electrolytes, and salivary flow rate—can aid in the detection, diagnosis, and management of dental caries.20–22 Antimicrobial peptides (cathelicidin peptide LL-37, defensins, histatins) protect against colonization of microbes. Major (mucins, proline-rich proteins, immunoglobins) and minor salivary glycoproteins (agglutinin, cystatins) help form the pellicle, which alters microbial colonization.21 However, current evidence demonstrating that amylase, lactoferrin, glycoproteins, agglutinin are suitable biomarkers for caries is limited, weak, and inconsistent. Some evidence shows that calcium, fluoride, bicarbonate, and phosphate have an anticariogenic effect when occurring in saliva. Furthermore, low salivary flow rate is a strong indicator for increased caries risk.22
Because caries is a multifactorial disease with contributing environmental and behavioral factors, using salivary diagnostics to determine caries risk is challenging. Dental caries prevalence is related to the quantities of the caries-causing bacteria Streptococcus mutans and Lactobacillus found in the saliva. A commercially available test can detect high titer levels of cariogenic pathogens. Moreover, saliva protects the teeth from caries via its antimicrobial agents, ability to buffer pH, and cleansing action.3
OTHER IMPLICATIONS
Some commercial marketed oral tests can detect biomarkers associated with the inflammatory response.2 Additionally, salivary biomarkers have been used to detect cancer in the lungs, breasts, and pancreas.14,23,24 Specifically, mRNA has been studied to detect lung cancer and its progression. In a study by Shang et al,23 carcinoembryonic antigen in the blood, a biomarker associated with monitoring cancer progression, and GREB1 and FRS2 biomarkers in saliva were measured to accurately detect nonsmall cell lung cancer. Likewise, MMP-9, which is associated with periodontal diseases, is linked to cardiovascular disease, cancer, multiple sclerosis, and neuropsychiatric disorders.8
COVID-19
In March 2020, the World Health Organization (WHO) labeled the outbreak of COVID-19, a severe acute respiratory syndrome, a global pandemic. Respiratory and oropharyngeal secretion biofluids contain COVID-19; salivary droplets and nasal discharge are the primary modes of transmission.25,26 Salivary diagnostics can detect COVID-19 through the virus’ RNA biomarkers.3 Diagnosis of COVID-19 is done by a nasopharyngeal swab to detect nucleic acid from the throat. This invasive procedure can induce coughing, which may increase the risk of transmission to the healthcare professional administering the test.25 In a study by Azzi et al,25 COVID-19 was identified in saliva and, interestingly, two subjects tested positive for COVID-19 using saliva samples but negative via a nasopharyngeal swab. This study suggests the virus may remain detectable in saliva and transmissible even when a nasopharyngeal swab test no longer provides a positive response. COVID-19 can be present in saliva via secretions from the upper and lower respiratory tract, GCF, and ducts of the salivary glands.27
POINT-OF-CARE TECHNOLOGY
Point-of-care technology evaluates biomarkers as indicators for an underlying disease or condition without central lab testing.28,29 Advancements in point-of-care technology allow early detection, diagnosis, and prognosis of oral and systemic diseases and cancer in a noninvasive manner with real-time results.
An emerging technology is biochip and electric field-induced release and measurement (EFIRM). A “lab-on-chip” technique combines laboratory procedures and a computer circuit to detect and diagnose multiple diseases at once.28 EFIRM is a highly sensitive electrochemical detection technique that uses biofluid, such as saliva, to detect biomarkers with results in approximately 30 minutes.29 Some point-of-care technology tests use saliva to detect periodontal pathogens, cariogenic bacteria, HPV genotyping, sexually transmitted diseases, inflammatory response, and COVID-19. Other tests use an oral rinse that is swished in the mouth for 30 seconds, followed by expectorating into a collection tube that is then mailed to the manufacturer’s laboratory for results. Additionally, some point-of-care technology identifies pathogens associated with periodontal diseases, whereas, other tests detect susceptibility to periodontal diseases by the detection of IL-6 or IL-1. However, they must be shipped to a lab and are not available for chairside results.2,29
Point-of-care technology can be used in a dental office to enhance individualized patient care by determining caries risk through the assessment of pathogens, the onset and progression of periodontal diseases, and screening for oral cancer. Point-of-care technology could help reduce the risk of transmission of COVID-19 in a convenient and cost-effective way. Providers could screen patients prior to performing aerosol-producing procedures to minimize risk of transmission.3 Although several commercial marketed oral tests are available, currently, there are no oral fluid tests approved by the US Food and Drug Administration to evaluate periodontal diseases, head and neck cancer, or dental caries.2
CONCLUSIONS
Saliva has gained attention in the medical and dental fields as an alternative for diagnostic testing and disease management. Saliva contains DNA, RNA, microRNA, and protein that can be easily collected, analyzed, and quantified. The information detected in oral fluid biomarkers can help reduce the burden of oral and systemic diseases. Early diagnosis is vital to an improved prognosis.
Dental hygienists are the prevention specialists in the dental field and are uniquely positioned to screen patients for systemic and oral diseases. The use of salivary diagnostics in the dental setting to help detect and monitor disease may expand interprofessional collaboration with medical colleagues and help oral health professionals to provide a more personalized approach to patient care. Salivary samples may become a routine specimen collected in dental and medical offices, similar to blood and urine samples.
REFERENCES
- Zhang L, Hua Xiao H, Wong DT. Salivary biomarkers for clinical applications. Mol Diagn Ther. 2009;13:245–259.
- American Dental Association. Salivary/Oral Fluid Diagnostics. Available at: ada.org/en/member-center/oral-health-topics/salivary-diagnostics. Accessed March 4, 2021.
- Santosh TS, Parmar R, Anand H, et al. A review of salivary diagnostics and its potential implication in detection of Covid-19. Cureus. 2020;12:1–10.
- Roi A, Rusu LC, Roi CI, et al. A new approach for the diagnosis of systemic and oral diseases based on salivary biomolecules. Dis Markers. 2019;2019:1–11.
- United States Centers for Disease Control and Prevention. Oral and Dental Health. Available at: cdc.gov/nchs/fastats/dental.htm. Accessed March 4, 2021.
- National Cancer Institute. Oral Cavity and Pharynx Cancer. Available at: seer.cancer.gov/statfacts/html/oralcav.html. Accessed March 4, 2021.
- Shah S. Salivaomics: the current scenario. J Oral Maxillofac Pathol. 2018;22:375–381.
- Korte DL, Kinney J. Personalized medicine: an update of salivary biomarkers for periodontal diseases. Periodontol 2000. 2016;70:26–37.
- Dawes C, Wong DTW. Role of saliva and salivary diagnostics in the advancement of oral health. J Dent Res. 2019;98:133–141.
- Lorenzo-Pouso AI, Pérez-Sayáns M, Bravo SB, et al. Protein-based salivary profiles as novel biomarkers for oral diseases. Dis Markers. 2018;2018:1–22.
- Wong DT. Salivaomics. J Am Dent Assoc. 2012;143:19–24.
- Kinney JS, Morelli T, Oh M, et al. Crevicular fluid biomarkers and periodontal disease progression. J Clin Periodontol. 2014; 41:113–120.
- Ghallab NA. Diagnostic potential and future directions of biomarkers in gingival crevicular fluid and saliva of periodontal diseases: review of the current evidence. Arch Oral Biol. 2018;87:115–124.
- Yoshizawa JM, Schafer CA, Schafer JJ, et al. Salivary biomarkers: toward future clinical and diagnostic utilities. Clin Microbiol Rev. 2013;26:781–791.
- Lee A, Ghaname CB, Braun TM, et al. Bacterial and salivary biomarkers predict the gingival inflammatory profile. J Periodontol. 2012;83:79–89.
- Jentsch HFR, Arnold N, Richter V, et al. Salivary, gingival crevicular fluid and serum levels of ghrelin and chemerin in patients with periodontitis and overweight. J Periodontal Res. 2017;5:1050–1057.
- Saxena S, Sankhla B, Sundaragiri KS, et al. A review of salivary biomarker: a tool for early oral cancer diagnosis. Adv Biomed Res. 2017;6:90.
- Kaur J, Jacobs R, Huang Y, et al. Salivary biomarkers for oral cancer and pre-cancer screening: a review. Clin Oral Investig. 2018;22:633–640.
- Santosh ABR, Jones T, Harvey J. A review on oral cancer biomarkers: understanding the past and learning from the present. J Cancer Res Ther. 2016;12:486–492.
- Martins C, Buczynski AK, Maiaa LC, et al. Salivary proteins as a biomarker for dental caries—a systematic review. J Dent. 2013;41:2–8.
- Hemadi AS, Huang R, Zhou Y, et al. Salivary proteins and microbiota as biomarkers for early childhood caries risk assessment. Int J Oral Sci. 2017;9:1–8.
- Gao X, Jiang S, Koh D, et al. Salivary biomarkers for dental caries. Periodontol 2000. 2016;70:128–141.
- Shang X, Zi H, Li Y, et al. Combined use of salivary biomarkers and carcinoembryonic antigen for lung cancer detection in a Chinese population. Medicine (Baltimore). 2019;98:1–7.
- Liu X, Yu H, Qiao Y, et al. Salivary glycopatterns as potential biomarkers for screening of early-stage breast cancer. EBioMedicine. 2018;28:70–79.
- Azzi L, Carcano G, Gianfagna F, et al. Salvia is a reliable tool to detect SARS-CoV-2. J Infect. 2020;80:1–6.
- Xu R, Cui B, Duan X, et al. Saliva: potential diagnostic value and transmission of 2019-nCoV. Int J Oral Sci. 2020;12:1–6.
- Sabino-Silva R, Jardim ACG, Siqueira WL. Coronavirus COVID-19 impacts to dentistry and potential. Clin Oral Investig. 2020;24:1619–1621.
- Khan RS, Khurshid Z, Asiri FYI. Advancing point-of-care (PoC) testing using human saliva as liquid biopsy. Diagnostics (Basel). 2017;7:1–12.
- Aro K, Wei F, Wong DT, et al. Saliva liquid biopsy for point-of-care applications. Front Public Health. 2017;5:1–9.
From Dimensions of Dental Hygiene. April 2021;19(4):26-28,31.