Risk Management in Pain Control
Follow these strategies to improve the safety of administering local anesthetic injections.
This course was published in the August 2012 issue and expires August 31, 2016. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Identify the new labeling of local anesthetic solutions and explain why the change was implemented.
- Calculate the maximum recommended dose in milligrams and cartridge number by using the suggested guidelines for local anesthetic and vasoconstrictor agents.
- Discuss the factors used to estimate the maximum recommended dose.
- Institute suggested medico-legal recommendations and documentation.
Fortunately for dental practitioners, this change does not alter the tried and true calculation formulas used for many years to determine correct dosage. It does, however, provide an impetus for reviewing proper risk management protocol for the use of local anesthesia, including dosage-related calculations, maximum recommended doses for different patients, potential adverse reactions, standards of care, and patient safety.
 CALCULATING DOSAGE
CALCULATING DOSAGE
Calculating the local anesthetic dose based on 1.8 mL provides a margin of safety, especially when used to estimate the maximum recommended dose (MRD).4 The MRD is the highest amount of an anesthetic drug(s) that can safely be administered per appointment when considering the individual’s age, weight, and health status.2–4 These estimates account for variations in patient responses to blood levels of each local anesthetic agent when the injection is administered by a skilled clinician, the drug is aspirated prior to depositing, and the agent is injected no faster than one cartridge per The MRD is used as a guide to determine the dosage of local anesthetic drugs and vasoconstrictors that can be safely administered to individual patients based on health status and treatment planned. To minimize adverse drug reactions, clinicians should always strive to administer the smallest effective dose of local anesthetic and vasoconstrictor necessary for the specific procedures being performed.4
Administering local anesthesia to children and older adults requires careful consideration due to their low body weight and blood volume. 4 Obese/morbidly obese children also require special attention. Based solely on weight, the MRD allows more anesthetic to be administered than may safely be biotransformed by an immature organ/liver system.3
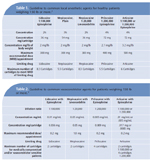
Although no published guidelines exist for obese children, dividing the typical maximum dose per pound of body weight in half may provide a safer estimation of the MRD. This reduced dosage is not a proven fail-safe formula, therefore, the amount administered should not approach the MRD.3 Dosage recommendations are listed on each manufacturer’s package insert. See Table 1 for generally accepted recommended dosage guidelines.5
VASOCONSTRICTORS
The drug in the solution (anesthetic agent or vasoconstrictor) that determines the MRD is called the limiting drug. For healthy individuals, the local anesthetic agent is generally the limiting drug. However, in lidocaine 2% with 1:50,000 epinephrine and for patients with a compromised medical status, the limiting drug is the vasoconstrictor. For medically compromised individuals, the maximum dose of vasoconstrictor should be limited to 0.04 mg epinephrine and 0.2 mg of levonordefrin per appointment. This reduces the number of cartridges with epinephrine 1:100,000 to two and 1:200,000 epinephrine to four. For 1:20,000 levonordefrin, the limit is two cartridges per appointment (Table 2).5
To calculate the MRD for local anesthetic drugs, clinicians need to know the amount or percentage of anesthetic agent and concentration of vasoconstrictor per cartridge, the maximum dose in milligrams per pound of body weight, and the MRD per appointment. Abiding by the absolute MRD for healthy individuals weighing more than 150 lb provides a safe guideline. Table 3 provides the calculation for determining the MRD for those who weigh less than 150 lb. If the patient is medically compromised, recommended dosages of the anesthetic drugs should be reduced.5
![]() PREVENTING ADVERSE AND OVERDOSE REACTIONS
PREVENTING ADVERSE AND OVERDOSE REACTIONS
Adverse and overdose reactions to amide anesthetics are usually caused by high plasma levels that affect the central nervous and cardiovascular systems.2–4 Negative reactions in the central nervous system are usually exhibited first. Adverse and overdose reactions most frequently occur when the MRD is approached, and especially when small children receive several doses during a single appointment. Careful patient monitoring and the use of consistent, meticulous aspiration technique are the keys to preventing overdose and adverse reactions. Individual doses must vary depending on the type of injection to be administered, the weight of a patient, and whether the patient is an adult, child, or medically compromised. The MRD should never be exceeded. Allergic reactions to amide anesthetics are rare but hypersensitivity or idiosyncratic and/or diminished tolerance may occur in a small number of patients. In the case of a negative reaction or overdose, clinicians must be prepared to manage a medical emergency, including maintaining the patient’s airway, evaluating the patient’s circulation, and recognizing the symptoms of seizure and/or convulsions.
STANDARDS OF CARE
All licensed dental professionals are obligated to provide care for patients in accordance with the standards of care, which are continually evolving as new research, technology, and materials become available. Risk reduction in local anesthesia administration includes excellent communication, obtaining informed consent, acting in a reasonable manner in the event of an adverse reaction or overdose, and accurate documentation of all care provided. Clinicians need to stay current with the literature, remain up to date on emergency training, and carefully maintain emergency equipment. Laws vary by state, but obtaining or updating an informed consent in writing prior to each appointment provides the greatest protection for clinicians. Informed consent should include an explanation of the advantages and potential risks and complications of treatment, treatment options, benefits or risk of no treatment, and associated fees, in addition to an opportunity for the patient to ask questions.4,6 Thoroughly reviewing the medical history and taking vital signs at each appointment are essential to preventing complications associated with local anesthesia administration. Consultation with other members of the patient’s health care team may be necessary if additional information regarding his or her medical status is needed.
In an increasingly litigious society, individuals can file civil or tort claims, as well as complaints with state licensing boards. As regulatory agencies do not require the plaintiff to carry the burden of proof, they may accept any information deemed relevant to the case. The local anesthetic treatment record, which documents all facets of the local anesthesia administration, will support clinicians in the event of a regulatory or legal challenge (Table 4).
LOCAL ANESTHETIC PACKAGE INFORMATION
Before a local anesthetic agent is administered, all manufacturer recommendations should be read and understood. Each insert contains valuable information.
All inserts include the manufacturer/ distributor of the drug; contact information; generic and trade drug names; concentration of the anesthetic agent (2%, 3%, 4%, or .05%); and vasoconstrictor and dilution ratios (1:20,000, 1:50,000, 1:100,000, or 1:200,000). Composition of the solution and the chemical structure will be included. The clinical pharmacology section may also contain the pharmacokinetic and pharmacodynamic properties (absorption, distribution, metabolism, excretion, mechanism of action, and clinical trial data). Package information will include indications and usage recommendations. Contraindications are also noted. Manufacturers will also print warnings that recommend clinicians be familiar with the diagnosis and management of potential emergencies. Resuscitative equipment, drugs, and oxygen should be immediately available. Local anesthetic agents can produce methemoglobinemia—a blood oxygenation deficiency in susceptible individuals. Initial signs include cyanosis of the skin, buccal mucous membranes, lips, and nail beds, as well as fatigue and weakness.2 Additional warnings relate to individuals with ischemic heart disease and the use of vasoconstrictors. Care should be taken to avoid intravascular injection by aspirating prior to injecting a local anesthetic solution. General precautions typically included in package inserts discuss the necessity of proper dosing and the use of skillful technique. Proper dosing involves administering the lowest dose that results in effective anesthesia for the procedure(s).
Age, weight, and medical status may necessitate reduced dosages and always require professional judgment. Debilitated, ill, or older adult patients and children should be administered doses appropriate for their unique characteristics. Monitoring vital signs is recommended prior to the administration of all local anesthetic drugs and during and after procedures as indicated by the individual’s health status. Caution should be used when inflammation and/or sepsis is present in the area of the injection site. Dosage recommendations should not be exceeded, and each insert refers clinicians to the dosage and administration section for additional information. Patients with cardiovascular and respiratory diseases must be monitored for central nervous system toxicity. Care should be taken with vasoconstrictors in patients who take tricyclic antidepressants (TCAs) due to the potential for hypersensitivity. Norepinephrine and levonordefrin should be avoided in patients taking TCAs.
One manufacturer’s insert discussed local anesthetics as possible triggers for inherited familial malignant hyperthermia (MH). MH is a syndrome where a rapid rise in temperature and severe muscle contractions occur that require early recognition and treatment to prevent harm.7 General anesthesia is a more common trigger, but controversy exists regarding whether an amide local anesthetic may act in a similar manner. Clinicians are advised to consult with physicians and/or the Malignant Hyperthermia Association of the United States prior to treating if patients report a history of MH. Careful monitoring of patients’ respiratory function, vital signs, and state of consciousness must be observed following each local anesthetic injection.
The “Information for Patients” section recommends that patients be informed in advance of the possibility of temporary sensation and/or muscle function loss following the administration of infiltration or nerve block injections. There are no well-controlled clinical studies in pregnant women, and it is unknown if local anesthetics are excreted in milk of nursing mothers. Healthy pregnant patients are classified as patients with mild systemic disease according to the American Society of Anesthesiology classification system (ASA II status). The second trimester is considered the safest period for mothers and fetuses, but if concerns arise, the patient’s health care team should be consulted prior to treatment.
Manufacturers also provide important information about sterilization, storage, and technical procedures. Local anesthetic cartridges should not be autoclaved but they can be disinfected with isopropyl alcohol (91%) or ethyl alcohol (71%). Cartridges should be stored in a dark place at temperatures below 77° F. Cartridges should be stored in the original box and not allowed to freeze. Cartridges should always be inspected prior to use and discarded if the plunger is extruded, cracks are evident, or the solution is discolored.2
CONCLUSION
It is the responsibility of all licensed oral care providers to adhere to recommended standards when administering local anesthetic drugs during dental treatment. Quality care requires a thorough review of the medical history, recording vital signs, being familiar with manufacturer inserts, and selecting the most appropriate anesthetic and technique for each individual patient and planned procedure(s). Calculating the MRD prior to the administration of any local anesthetic drug is the standard of care and is necessary for patient safety. Clinicians must strive to stay abreast of current evidence, products, technologies, and techniques.
REFERENCES
- US Food and Drug Administration. 21 CFR Parts 201, 314, and 601. Available at:www.fda.gov/ohrms/dockets/98fr/06-545.pdf. Accessed July 10, 2012.
- Logothetis DD. Local Anesthesia for the Dental Hygienist. St Louis: Elsevier; 2012.
- Bassett KB, DiMarco AC, Naughton DK. Local Anesthesia for Dental Professionals. Upper SaddleRiver, NJ: Pearson; 2010.
- Malamed SF. Handbook of Local Anesthesia. 6th ed. St Louis: Elsevier; 2013.
- Paarmann C, Royer R. Pain Control for Dental Practitioners. Baltimore: Lippincott Williams &Wilkins; 2008.
- Orr DL II, Curtis WJ. Obtaining informed consent for the administration of local anesthetic indentistry. J Am Dent Assoc. 2005;136:1568–1571.
- Minasian A, Yagiela JA. The use of amide local anesthetics in patients susceptible to malignanthyperthermia. Oral Surg Oral Med Oral Pathol. 1988;66:405–415.
From Dimensions of Dental Hygiene. August 2012; 10(8): 24-27.