
Nonfluoride Caries Prevention
The American Dental Association Council of Scientific Affairs provided a systematic review and evidence-based recommendations for the use of products outside of the fluoride-based armamentarium.
This course was published in the December 2012 issue and expires December 2015. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the process conducted to create the report on nonfluoride caries preventive agents by the American Dental Association Council of Scientific Affairs.
- Describe the expert panel’s recommendations for the use of nonfluoride caries prevention agents.
- Identify suggestions for future research subjects.
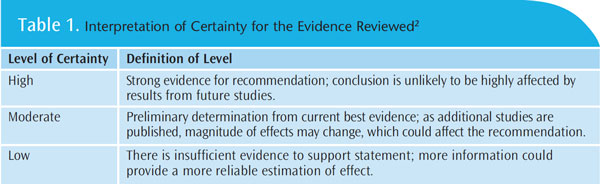
In 2011, the American Dental Association (ADA) Council on Scientific Affairs formed an expert panel of researchers and clinicians to conduct a systematic review (SR) and meta-analysis (MA) to evaluate the evidence regarding nonfluoride products for caries prevention.1 Recognizing the well-documented and evidence-based primary caries prevention strategies—fluoride, pit and fissure sealants, and dietary improvements—the panel provided evidence-based recommendations for adjunctive use of nonfluoride products in clinical care.1,2 These included xylitol, chlorhexidine, casein derivatives, and others. Research on each product category was graded for certainty and defined as high, moderate, or low based on a standardized grading system (Table 1). Evidence for efficacy was weighted against potential adverse events reported in trials. When the panel was unable to reach a consensus, a majority vote determined the final recommendation.
STUDY DESIGN
 The randomized controlled trial (RCT) is the study design with the least risk of bias. The panel included 71 published studies, 50 of which were RCTs. Only six of the studies used were conducted in the United States. Overall, the published studies examined lacked good quality trials. For example, many did not follow the Consolidated Standards of Reporting Trials (CONSORT) guidelines regarding proper randomization, allocation concealment of groups, account for losses to follow-up, and intention to treat analyses.3 Many studies did not provide information on the caries risk status of subjects, and there was a lack of uniformity in the description of subjects’ fluoride exposures. Due to these deficiencies, the panel concluded nonfluoride preventive agents should be considered as adjunctive agents for caries control, with fluoride, pit and fissure sealants, and proper nutrition remaining the first choices in treatment. Evidence did not indicate all agents were effective, but some should be considered in patients who do not respond well to the aforementioned strategies and those at greatest caries risk. Because none of the studies contained pregnant women, the recommendations do not apply to this patient population.1
The randomized controlled trial (RCT) is the study design with the least risk of bias. The panel included 71 published studies, 50 of which were RCTs. Only six of the studies used were conducted in the United States. Overall, the published studies examined lacked good quality trials. For example, many did not follow the Consolidated Standards of Reporting Trials (CONSORT) guidelines regarding proper randomization, allocation concealment of groups, account for losses to follow-up, and intention to treat analyses.3 Many studies did not provide information on the caries risk status of subjects, and there was a lack of uniformity in the description of subjects’ fluoride exposures. Due to these deficiencies, the panel concluded nonfluoride preventive agents should be considered as adjunctive agents for caries control, with fluoride, pit and fissure sealants, and proper nutrition remaining the first choices in treatment. Evidence did not indicate all agents were effective, but some should be considered in patients who do not respond well to the aforementioned strategies and those at greatest caries risk. Because none of the studies contained pregnant women, the recommendations do not apply to this patient population.1
EVALUATION OF THE EVIDENCE
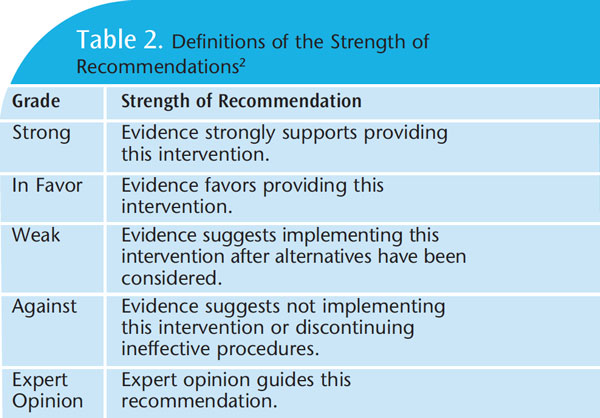
The new guidelines provide an executive summary of findings regarding the efficacy of nonfluoride agents in reducing caries and arresting or reversing the progression of caries.2 Nonfluoride agents, such as xylitol, varnishes with chlorhexidine, amorphous calcium/casein products, and others, were presented in a table with designations as “strong,” “in favor,” “weak,” “against,” or those without a significant body of evidence were designated as “expert opinion” (Table 2). The summary also explains that recommendations are not intended to define a standard of care and should be integrated with the practitioner’s professional judgment, patient needs, and patient preferences.1,2 The ADA expert panel evaluated the research for various types of products and their efficacy on coronal caries or root caries. When studies were similar, data were combined in a MA to improve the validity of information.1
SUCROSE-FREE POLYOL CHEWING GUMS
The committee reviewed 15 studies comparing subjects who chewed sucrose-free poly gum (eg, sorbitol only, xylitol only, or polyol combinations) to subjects who did not chew gum. The trials considered were all conducted in children between 5 and 13. Gum chewing was supervised, in most studies, with the frequency of chewing between two times and six times per day, and the duration of chewing ranging from 10 minutes to 20 minutes. Only nine of the 15 studies were included in the MA due to incomplete reporting of data or comparisons in some studies to noncomparable outcome measures.
The results of the MA indicated there is a statistically significant reduction in caries with the use of all sucrose-free polyol gums when compared to no gum chewing. Subgroup analysis showed that xylitol gum had the highest caries reduction, followed by gums with a combination of polyols. Further analysis found that xylitol gum was more effective in reducing the incidence of caries than sorbitol gum. Overall, the panel concluded with moderate certainty that “in children age 5 to 16, supervised consumption of chewing gum with sucrose-free polyol (xylitol only or polyol combinations) for 10 minutes to 20 minutes after meals may reduce the incidence of coronal caries.”2 This recommendation is noted as “weak.” The same recommendation was made for adults but the category of evidence was designated as “expert opinion” (Table 2).2
The panel also considered adverse impacts of gum chewing. Among children younger than 5 and those with chewing or swallowing disorders, neurologic impairments, and/or behavioral factors that might increase the risk of choking, the safety of gum chewing should be considered. The majority of the panel agreed that the benefits of supervised gum chewing along with a primary caries prevention routine, especially in children at high risk of caries, outweigh potential adverse effects.1,2
XYLITOL CANDY AND LOZENGES
Four studies that evaluated caries reduction with the use of xylitol candy/lozenges/tablets were reviewed by the panel. Combined results from three of the studies found a statistically significant effect in favor of xylitol lozenge/candy. A majority of the panel recommended the use of xylitol lozenges or hard candy after meals for children older than 5. The suggested
XYLITOL SYRUP
The panel also reviewed one study conducted on children younger than 2 that compared the use of a syrup form of xylitol vs a control syrup. Although the study found statistically significant differences in favor of xylitol syrup, the panel concluded “there is insufficient evidence that xylitol syrup prevents caries in children under 2.”1 The panel did not suggest the syrup form of xylitol was ineffective, only that there was insufficient evidence to support a recommendation.
XYLITOL DENTIFRICE
Two large-scale studies that compared dentifrice with 10% xylitol in addition to fluoride vs fluoride dentifrice without xylitol were examined. Based on these studies, the panel was unable to make a determination for or against the effect of xylitol in caries reduction when added to a dentifrice. The panel concluded “there is insufficient evidence that xylitol in dentifrices prevents caries.”1
CHLORHEXIDINE VARNISH
The federal Food and Drug Administration has not approved chlorhexidine (CHX) for caries prevention, although CHX is marketed in a variety of concentrations and delivery methods in Europe and Canada. The panel identified 24 studies relating to various CHX products including varnishes, gels, and rinses.
The panel examined five RCTs evaluating the anti-caries effectiveness of CHX varnishes. The specific CHX used as a single agent in the five studies is not on the market in the US. The only CHX varnish currently available is a 1% CHX/1% thymol combination varnish. Studies performed in Europe varied in design with CHX compared to a variety of agents. Some studies used a comparison group that received a placebo varnish. The subjects used in the studies were preschool, school-age, and adolescent children. The MA revealed nonsignificant differences between groups, which the panel decided was evidence for lack of effect. Therefore, the panel had moderate certainty for concluding “in children age 4 to 18, professionally applied 10% to 40% CHX varnish does not reduce the incidence of caries.”2 The panel also examined one RCT conducted on 40% CHX varnish that showed statistically significant root caries reduction in adults. The study was judged to be of poor quality, and thus, the panel concluded: “in adults, there is insufficient evidence that use of 40% CHX varnish reduces the incidence of root caries.”1
The panel found six studies evaluating the efficacy of a 1:1 mixture of CHX/thymol varnish. The MA included more than 700 subjects and showed a nonsignificant difference between groups. The panel determined, with low certainty, due to the poor quality of most studies, that “in children up to 15, application of a 1:1 mixture of chlorhexidine/thymol varnish alone or in combination with fluoride does not reduce the incidence of coronal caries.”2
The panel examined three RCTs evaluating the effectiveness of 1:1 chlorhexidine/thymol varnish on adult root caries. All of the studies showed a statistically significant improvement in caries status and led the panel to conclude with moderate certainty that “in adults and elderly people, application of a 1:1 mixture of chlorhexidine/ thymol varnish placed every 3 months reduces the incidence of root caries.”1
CHLORHEXIDINE RINSE
Four RCTs evaluating the effects of a 0.12% CHX mouthrinse on coronal caries were examined. Based on these four RCTs of fair to good quality and the results of the MA, which showed a nonsignificant difference, the panel concluded with high certainty that “in children and adults, use of 0.05% to 0.12% chlorhexidine rinse does not reduce the incidence of coronal caries.”1
Two other RCTs examined the incidence of root caries only in adults and older adults. Both studies found that the use of 0.12% CHX rinse did not statistically decrease the incidence of root caries. Based on the two trials, the panel concluded with moderate certainty: “in adults and elderly people, use of 0.12% chlorhexidine rinse, alone or in combination with fluoride, does not reduce the incidence of root caries.”1
CHLORHEXIDINE GEL
The panel evaluated five pediatric studies that studied the efficacy of CHX gels. Three studies were judged to be of fair quality and three of the five reported a nonsignificant difference in outcome. Due to the different delivery methods, the panel did not incorporate the results for MA. The panel was unable to make a determination on the efficacy of 1% CHX gel for caries and concluded: “in children aged 3 to 15, there is insufficient evidence that professionally applied 0.5% to 1% CHX gel reduces the incidence of coronal or root caries.”1,2 Most clinical study investigations that examined the use of various CHX formulations to reduce coronal caries did not find a statistically significant reduction with the use of any vehicle. As a result, the panel did not recommend using CHX products for coronal caries prevention at this time. However, the panel acknowledged that application of 1:1 chlorhexidine/thymol varnish may help reduce the incidence of root caries in adults and older adults. There is not enough evidence to support the use of 10% to 40% CHX varnish, alone or with fluoride, for reduction of coronal caries.
AMORPHOUS CALCIUM AND CASEIN DERIVATIVES
Various casein derivative agents are being added to toothpastes or other mediums to promote remineralization to damaged tooth structure. The panel examined nine studies that evaluated various calcium- and/or phosphate-containing agents with and without casein derivatives. Two studies were of good quality, five were of fair quality, and the rest were of poor quality. All studies except one were RCTs. Due to differences in the study designs, the panel was unable to combine data into a MA. The panel concluded, “there is insufficient evidence from clinical trials that use of agents containing calcium and/or phosphate with or without casein derivatives lowers the incidence of either coronal or root caries.”1
IODINE
The panel examined four RCTs that evaluated 10% povidone-iodine on coronal caries in preschool and school-age children. All of the studies included a small number of subjects and study design differences did not allow data to be combined in a MA. The panel concluded: “there is insufficient evidence that the use of iodine lowers the incidence of caries.”1
OTHER PRODUCTS
No clinical recommendations were provided on triclosan, an antibacterial agent, or sialogogues, drugs or substances that increase salivary flow rate. The panel found no published literature evaluating either the effects of triclosan or the effects sialogogues on caries prevention.
CONCLUSIONS
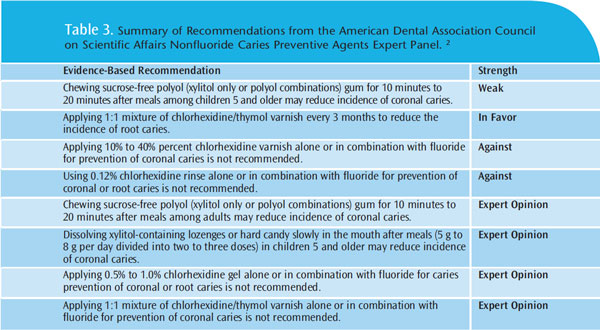
A summary of the expert panel’s recommendations appears in Table 3.2 These guidelines are dynamic and will be updated when new evidence emerges. The panel asserted that their guidelines are limited by the dearth of evidence conducted on prevention in the US. It suggests that additional research be undertaken to better understand nonfluoride caries preventive agents. Specifically, the panel recommends that more RCTs that adhere to the CONSORT guidelines and standardize reporting by age, dentition, and caries risk status are needed. The panel suggests investigating a variety of hypotheses, including optimal mode of delivery, dosage, frequency, duration, and adverse effects of xylitol when used to prevent caries among adults, older adults, and patients with special needs with different levels of fluoride exposure; efficacy of nonfluoride preventive agents when used in concert with fluoride to prevent caries among older adults and those with special needs; effectiveness of nonfluoride agents designed to prevent demineralization or promote remineralization on caries prevention among children and adults; efficacy of iodine, triclosan, and other antimicrobial agents on caries prevention among children and adults; and effectiveness of products to decrease the incidence of root surface lesions.2 The panel also suggests that new technologies may provide additional strategies for preventing dental caries, including arginine, probiotics, and targeted biomolecules.2 Once rigorous research studies have been conducted and published on these and other possible therapies, the Scientific Council will begin another review of nonfluoride caries preventive products.2
It is important to note that just because an intervention hasn’t been shown through high-level science to be of great value doesn’t mean it’s valueless. The interventions evaluated in this systematic review, especially those supported by at least some evidence, may have roles in helping manage caries in some patients, especially those living in areas without community water fluoridation and those with limited or no access to professional oral care. Among both high-risk children and older adults with exposed dentin and/or decreased salivary function, nonfluoride interventions may be useful adjuncts to well-demonstrated anti-caries interventions. A patient’s caries risk status along with professional judgement and the needs of the patient should guide professional decision making.
SUMMARY
In the effort to prevent, reduce, and control caries, oral health care providers have many strategies to consider. This review intended to increase understanding of the nonfluoride options and when to incorporate them into patient care. The ADA also provides guidelines for topical application of fluoride products for children and adults.4 As evidence-based decision making asserts, clinicians must consider the scientific evidence, clinical/patient circumstances, their own experience and judgement, and patient preferences or values. Incorporating solid scientific evidence, such as provided by the expert panel’s report, is an important facet to providing the best possible patient care.
References
- American Dental Association, Council on Scientific Affairs, Center for Evidence Based Dentistry. Non-fluoride caries preventive agents: Full report of a systematic review and evidence basedrecommendations. Available at: www.ebd.ada.org/contentdocs/ clinical_recommendations_non_fluoride_caries_preventive_agents_full_report.pdf. Accessed November 26, 2012.
- Rethman MP, Beltran-Aguilar ED, Billings RJ, et al. Nonfluoride caries-preventive agents: executive summary of evidence-based clinical recommendations. J Am Dent Assoc.
- Altman DG, Barbour V, Berlin JA, et al. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–732.
- American Dental Association Council on Scientific Affairs. Professionally applied topical fluoride: evidence-based clinical recommendations. J Am Dent Assoc. 2006;137:1151–1159.
From Dimensions of Dental Hygiene. December 2012; 10(12): 40–43.



