
What the Future Holds For Periodontal Treatment
Although today’s periodontal treatment has experienced significant advancement, even more promising therapies are on the horizon.
One of periodontology’s greatest strengths is that it is not static; rather, it continues to evolve and readjust its focus on the basis of contemporary research. Table 1 lists the topics currently under investigation. This review will discuss some, but not all, of the developments that may help reshape periodontal therapy over the next 10 years.
Accurate assessment of individual risk is considered one of the most important aspects of periodontal examination, diagnosis, prognosis, and treatment planning. To date, this has been elusive, but, nonetheless, it will remain an important aspect of research and practice in the years to come. Recognition that periodontitis is a multifactorial disease involving a complex interplay between host, environment, and microbes is central to current scientific understanding.1 While it is well accepted that the development of periodontitis is intimately associated with the bacteria residing in the subgingival biofilm, there are some patients who, despite the presence of considerable plaque deposits, do not develop periodontitis. Conversely there are individuals who develop advanced and destructive forms of periodontitis, yet appear to have minimal deposits of plaque. It seems that although plaque is necessary for periodontitis to develop, it alone is insufficient for the development of periodontal diseases.2,3 Indeed, it is now evident that in addition to plaque, other risk factors—such as opportunistic pathogens, environmental influences, genetics, host responses, connective tissue metabolism, and bone metabolism—are critical to the clinical manifestation of periodontitis (Figure 1).
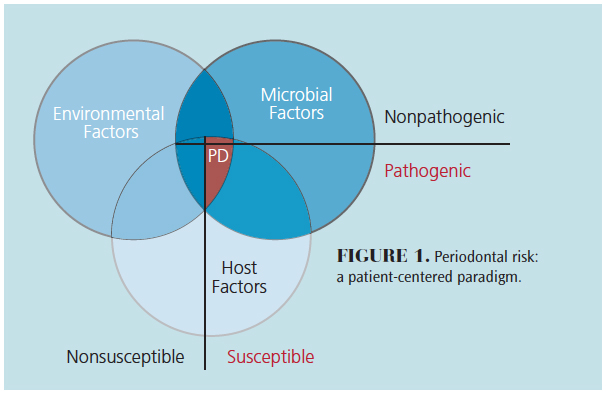
Understanding this multifactorial nature has led to the development of a more informed management process. Such processes dictate that, in addition to recognizing the fundamental role of oral hygiene, other risk factors that contribute to periodontitis must be controlled in order for treatment to be successful.4,5 For example, plaque appears to account for only 20% of the risk for developing periodontitis.6 Therefore, there must be another 80% of risk elements (ie, modifying and predisposing factors) that must be considered when assessing a patient’s periodontal condition. This can be thought of as the personalized medicine approach to diagnosing and treating periodontal diseases.7
In the future, identifying individual risk factors within the paradigm of personalized medicine will become central to periodontal practice. In this context, the emerging field of genomics8,9 will play an increasingly important role. Furthermore, completion of the human genome project now allows the use of sophisticated approaches—such as genome-wide screening of populations—to determine the genetic contributions to periodontitis in its various forms.10 However, genetics alone cannot fully account for the etiology and progression of the disease process.11
Epigenetics is the study of cellular and physiological phenotypic trait variations caused by external or environmental factors that switch genes on and off.12 Clearly, this has great potential to impact scientific understanding of periodontitis and how environment and genes interact in the clinical manifestation of disease.13,14 However, while it seems entirely probable this extra genetic material may have a great deal of interplay with disease, at present, its role is poorly understood.
HOST-PATHOGEN RELATIONSHIPS
Understanding the central role of the host response in the development of periodontitis opens new avenues for patient management. These will not replace mechanical subgingival debridement, but will serve as adjuncts to traditional approaches to improve and hasten treatment outcomes. Such therapies aimed at controlling the host inflammatory response will also result in modification of the subgingival environment and, thus, significantly influence the microbial ecology.
The proposal of the “ecological plaque hypothesis” in 2003 was a significant development in the conceptual understanding of the subgingival environment and how the local environment can influence bacterial composition.15,16 However, this hypothesis was poorly embraced because it was considered discordant with conventional periodontal microbiology concepts that dictated specific bacteria were solely responsible for periodontitis.17 Nonetheless, the concept that host modification of the periodontal pocket whereby inflammatory conditions select for so-called pathogenic species through a process known as “reciprocal interaction” was eventually embraced.18 The process by which the host can modify the subgingival bacterial environment has significant ramifications for future host modulation based on controlling the inflammation, rather than focussing solely on eliminating infection.2,19–21
The subgingival microbiota cannot be overlooked. Through the unravelling of the human microbiome, how microbial communities interact furthers understanding of the periodontal microbiota and its role in the development and progression of periodontitis in a new light.22 Recently, the concept of bacterial dysbiosis as a key player in the pathogenesis of periodontitis has been suggested.23,24 In this model, changes within the subgingival milieu cause microbial shifts and imbalances, resulting in the overgrowth of pathogenic microbes.25 This concept supports the value of host modulation therapy as a viable adjunct.
PERIODONTAL REGENERATION
Regeneration of tissues damaged by periodontitis has long been considered a goal of periodontal treatment. Numerous strategies have been investigated to achieve periodontal regeneration, such as root surface conditioning or implantation of bone substitutes into periodontal defects. Yet, each of these procedures remains technique sensitive and clinically unpredictable. This is due, in part, to a lack of understanding of the requirements for periodontal regeneration—namely, the encouragement the regrowth of new cementum, bone, and periodontal ligament. Nonetheless, the profession as become focused on filling holes in bone rather than studying the natural healing processes required to regenerate the periodontal attachment apparatus.26 A lack of clarity regarding the contribution of the various tissue components in periodontal wound healing explains the widespread misuse of bone transplantation in the treatment of intrabony pockets.27
Periodontal regeneration must be founded on sound biologic principles.28 By applying biologically sound principles to the problem of periodontal regeneration, the clinical procedure of guided tissue regeneration was developed in the 1980s.29 At the time, this was a significant advance. However, it soon became apparent that periodontal regeneration was clinically difficult to achieve on a reliable basis due to a vast range of patient and operator variables.30 More recently, the application of biological agents onto root surfaces has induced significant regeneration of damaged periodontal tissues.31 An advantage is that biological agents are simpler to use and provide comparable and sometimes superior results.32 The regenerative capacity of biologic agents depends on many variables.
In recent years, implantation of mesenchymal stem cells into periodontal defects has been investigated as a means of inducing periodontal regeneration.33 The first report about the isolation and characterization of periodontal ligament stem cells was published in 2004, and since then the field of dental stem cells has advanced quickly, particularly with regard to its potential use for periodontal regeneration.34–37 Another development is the use of gene therapy for periodontal regeneration. However, this technology requires sophisticated technical manipulation of cells and results in considerable caries morbidity issues.38,39 In order for these cell-based regenerative therapies to be successful, suitable delivery devices will need to be developed so the cells can be successfully delivered to the sites of interest. The development of biocompatible scaffolds will be crucial in the next phases of this technology.40 Indeed, this is an active area of research involving the development of biodegradable materials, smart materials and three-dimensional bioprinting.41–45 While the field of cell-based tissue engineering for periodontal regeneration is exciting and the future looks promising, considerable work must be done before such modalities can become a reliable and predictable approach to treatment.
PERIODONTAL MEDICINE
The term “periodontal medicine” was coined in 1996.46 The field generally encompasses the interactions between periodontal health and disease, and systemic health and disease. The defining concept is that periodontal inflammation and the periodontal microbiome interact, resulting in a significant increase in the overall level of systemic inflammation. In turn, this influences the incidence, severity, and progression of chronic inflammatory conditions. Furthermore, there may be a bidirectional relationship between periodontitis and some systemic conditions, in which periodontitis can affect systemic health—and the opposite may also occur. The interest in periodontal/systemic relationships has resulted in the number of conditions associated with periodontitis growing to unrealistic levels. A literature search will reveal an increasing number of reports of associations that have poor biologic plausibility and lack critical scientific assessment. Indeed, there are more than 100 periodontal/systemic associations reported in the literature, most of which are observational studies based on sources of very low evidence. There are many levels of evidence that vary in significance (Figure 2), ranging from so-called expert opinion (lowest form) to systematic reviews and randomized clinical trials (highest forms). When evaluating the literature, the strength of the evidence presented must be considered.
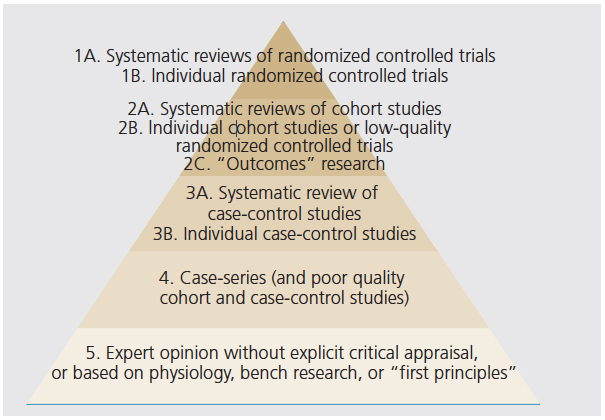
FIGURE 2. Levels of evidence.
Another issue is causality vs association. While associations can be made easily, causality is a far more complex problem. Hill’s47 criteria was developed to help determine causality (see the web version for more information). Per these criteria, it appears unlikely that periodontitis is causal of other conditions. However, disease associations may be just as important because they allow researchers to identify individuals who are likely to aggregate with common conditions and underlying pathologic processes.
The emergence of periodontal medicine as a subdiscipline of periodontology has resulted in a new direction in the understanding of periodontal disease. Classical periodontal therapy has been focused only within the oral environment to preserve or restore the structure, function, and esthetics of the dentition. However, science’s current understanding that periodontal inflammation and infection may be intricately associated with other chronic systemic conditions has led to a new focus directed to whole body health, and managing the negative effects of periodontitis on systemic health.
While the emergence of periodontal medicine is important, clinicians and researchers must be mindful to focus only on those associations that have been established through sound scientific investigation. At a joint meeting of the American Academy of Periodontology and European Federation of Periodontology, it was concluded there was evidence in the literature to support the concept that periodontitis is associated with some, but not all, of the diseases and conditions reviewed.49 Currently, the conditions with the strongest evidence for association with periodontitis are cardiovascular disease, diabetes mellitus, obesity, metabolic syndrome, and rheumatoid arthritis.
CONCLUDING COMMENTS
While there are many important topics that could be addressed, the ones selected for this review illustrate both the challenges facing periodontology, and the robust nature of this specialty. These topics may significantly impact prevention, diagnosis, and management of one of the most common chronic inflammatory conditions afflicting humans. As periodontology continues to evolve, one thing is clear: the future is exciting.
BONUS WEB CONTENT

REFERENCES
- Genco RJ, Borgnakke WS. Risk factors for periodontal disease. Periodontol2000. 2013;62:59–94.
- Bartold PM, Van Dyke TE. Periodontitis: a host-mediated disruption ofmicrobial homeostasis. Unlearning learned concepts. Periodontol 2000.2013;62:203–217.
- Clarke NG, Hirsch RS. Personal risk factors forgeneralized periodontitis. J Clin Periodontol. 1995;22:136–145.
- Braun TM, Doucette-Stamm L, Duff GW, Kornman KS,Giannobile WV. Counterpoint: risk factors, including genetic information, addvalue in stratifying patients for optimal preventive dental care. J Am DentAssoc. 2015;146:174–178.
- Lang NP, Suvan JE, Tonetti MS. Risk factorassessment tools for the prevention of periodontitis progression a systematicreview. J Clin Periodontol. 2015;42(Suppl 16):S59–S70.
- Grossi SG, Zambon JJ, Ho AW, et al. Assessment ofrisk for periodontal disease. I. Risk indicators for attachment loss. JPeriodontol. 1994;65:260–267.
- Kornman KS, Duff GW. Personalized medicine: willdentistry ride the wave or watch from the beach? J Dent Res.2012;91(Suppl 7):8S–11S.
- Grant MM. What do ‘omic technologies have tooffer periodontal clinical practice in the future? J Periodontal Res.2012;47:2–14.
- Trindade F, Oppenheim FG, Helmerhorst EJ, AmadoF, Gomes PS, Vitorino R. Uncovering the molecular networks in periodontitis. ProteomicsClin Appl. 2014;8:748–761.
- Vaithilingam RD, Safii SH, Baharuddin NA, et alMoving into a new era of periodontal genetic studies: relevance of largecase-control samples using severe phenotypes for genome-wide associationstudies. J Periodontal Res. 2014;49:683–695.
- American Dental Association Council on ScientificAffairs. Genetics and oral health. Available at:ada.org/en/member-center/oral-health-topics/genetics-and-oral-health. AccessedOctober 3, 2017.
- Barros SP, Offenbacher S. Epigenetics: connectingenvironment and genotype to phenotype and disease. J Dent Res.2009;88:400–408.
- Barros SP, Offenbacher S. Modifiable risk factorsin periodontal disease: epigenetic regulation of gene expression in theinflammatory response. Periodontol 2000. 2014;64:95–110.
- Larsson L, Castilho RM, Giannobile WV.Epigenetics and its role in periodontal diseases: a state-of-the-art review. JPeriodontol. 2015;86:556–568.
- Marsh PD, Head DA, Devine DA. Ecologicalapproaches to oral biofilms: control without killing. Caries Res. 2015;49(Suppl1):46–54.
- Marsh PD. Dental diseases-are these examples ofecological catastrophes? Int J Dent Hyg. 2006;4(Suppl 1):3–10.
- Feres M, Cortelli SC, Figueiredo LC, Haffajee AD,Socransky SS. Microbiological basis for periodontal therapy. J Appl Oral Sci.2004;12:256–266.
- Socransky SS, Haffajee AD. Periodontal microbialecology. Periodontol 2000. 2005;38:135–187.
- Gokhale SR, Padhye AM. Future prospects ofsystemic host modulatory agents in periodontal therapy. Br Dent J.2013;214:467–471.
- Elavarasu S, Sekar S, Murugan T. Host modulationby therapeutic agents. J Pharm Bioallied Sci. 2012;4(Suppl 2):S256–S259.
- Hasturk H, Kantarci A, Van Dyke TE. Paradigmshift in the pharmacological management of periodontal diseases. Front OralBiol. 2012;15:160–176.
- Darveau RP. Periodontitis: a polymicrobialdisruption of host homeostasis. Nat Rev Microbiol. 2010;8:481–490.
- Hajishengallis G. Periodontitis: from microbialimmune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44.
- Pérez-Chaparro PJ, Gonçalves C, Figueiredo LC, etal. Newly identified pathogens associated with periodontitis: a systematicreview. J Dent Res. 2014;93:846–858.
- Darveau RP, Hajishengallis G, Curtis MA. Porphyromonasgingivalisas a potential community activist for disease. JDent Res. 2012;91:816–820.
- Becker W, Becker BE. Periodontal regeneration: acontemporary reevaluation. Periodontol 2000. 1999;19:104–114.
- Karring T, Nyman S, Lindhe J, Sirirat M.Potentials for root resorption during periodontal wound healing. J ClinPeriodontol. 1984;11:41–52.
- Bartold PM, McCulloch CA, Narayanan AS, Pitaru S.Tissue engineering: a new paradigm for periodontal regeneration based onmolecular and cell biology. Periodontol 2000. 2000;24:253–269.
- Karring T, Nyman S, Gottlow J, Laurell L.Development of the biological concept of guided tissue regeneration — animaland human studies. Periodontol 2000. 1993;1:26–35.
- Reynolds MA, Kao RT, Camargo PM, et al.Periodontal regeneration — intrabony defects: a consensus report from the AAPRegeneration Workshop. J Periodontol. 2015; 86(Suppl 2):S105–S107.
- Stavropoulos A, Wikesjö UM. Growth anddifferentiation factors for periodontal regeneration: a review on factors withclinical testing. J Periodontal Res. 2012;47:545–553.
- Koop R, Merheb J, Quirynen M. Periodontalregeneration with enamel matrix derivative in reconstructive periodontaltherapy: a systematic review. J Periodontol. 2012;83:707–720.
- Bassir SH, Wisitrasameewong W, Raanan J, et al.Potential for stem cell-based periodontal therapy. J Cell Physiol.2016;231:50–61.
- Seo BM, Miura M, Gronthos S, et al. Investigationof multipotent postnatal stem cells from human periodontal ligament. Lancet.2004;364:149–155.
- Bartold PM, Gronthos S. Standardization ofcriteria defining periodontal ligament stem cells. J Dent Res.2017;96:487–490.
- Zhu W, Liang M. Periodontal ligament stem cells:current status, concerns, and future prospects. Stem Cells Int.2015;2015:972313.
- Hynes K, Menicanin D, Gronthos S, Bartold PM.Clinical utility of stem cells for periodontal regeneration. Periodontol2000. 2012;59:203–227.
- Rios HF, Lin Z, Oh B, Park CH, Giannobile WV.Cell- and gene-based therapeutic strategies for periodontal regenerativemedicine. J Periodontol. 2011;82:1223–1237.
- Su F, Liu SS, Ma JL, Wang DS, E LL, Liu HC.Enhancement of periodontal tissue regeneration by transplantation ofosteoprotegerin-engineered periodontal ligament stem cells. Stem Cell ResTher. 2015;6:22.
- Bartold PM, Xiao Y, Lyngstaadas SP, Paine ML,Snead ML. Principles and applications of cell delivery systems for periodontalregeneration. Periodontol 2000. 2006;41:123–135.
- Rasperini G, Pilipchuk SP, Flanagan CL, et al.3D-printed bioresorbable scaffold for periodontal repair. J Dent Res.2015;94(Suppl 9):153S–157S.
- Bartold PM, Gronthos S, Ivanovski S, Fisher A,Hutmacher DW. Tissue engineered periodontal products. J Periodont Res.2016;51:1–15.
- Obregon F, Vaquette C, Ivanovski S, Hutmacher DW,Bertassoni LE. Three-dimensional bioprinting for regenerative dentistry andcraniofacial tissue engineering. J Dent Res. 2015;94(Suppl 9):143S–152S.
- Ivanovski S, Vaquette C, Gronthos S, HutmacherDW, Bartold PM. Multiphasic scaffolds for periodontal tissue engineering. JDent Res. 2014;93:1212–1221.
- Park CH, Kim KH, Rios HF, Lee YM, Giannobile WV,Seol YJ. Spatiotemporally controlled microchannels of periodontal mimicscaffolds. J Dent Res. 2014;93:1304–1312.
- Offenbacher S. Periodontal diseases:pathogenesis. Ann Periodontol. 1996;1:821–878.
- Hill AB. The environment and disease: associationor causation? Proc R Soc Med. 1965;58:295–300.
- Bartold PM, Mariotti A. The future ofperiodontal-systemic associations: raising the standards. Curr Oral HealthRep. 2017;4:258–262.
- Linden GJ, Lyons A, Scannapieco FA. Periodontalsystemic associations: review of the evidence. Jointly published in: J ClinPeriodontol. 2013;40(Suppl 14):S8–S19. J Periodontol. 2013;84(Suppl4):S8–S19
Featured photo by 4X6/ISTOCK/GETTY IMAGES PLUS
From Dimensions of Dental Hygiene. December 2017;15(12):14,16-18.


