 DIMASOBKO/iSTOCK/THINKSTOCK
DIMASOBKO/iSTOCK/THINKSTOCK
Treating Patients on Antiplatelet Therapy
In order to safely and effectively manage this patient population, oral health professionals should be able to recognize the medical indications for which antiplatelets are prescribed and understand their adverse effects.
This course was published in the January 2017 issue and expires January 2020. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Describe the mechanism of action for antiplatelet medications.
- Identify the most commonly used antiplatelet therapies.
- Discuss the implications of antiplatelet therapy on oral health care delivery.
Aspirin was the first oral antiplatelet therapy. In addition to antiplatelet effects, aspirin provides analgesic, anti-inflammatory, and antipyretic effects. Aspirin still has a viable place in treating patients with a variety of vascular disorders and preventing ischemic stroke or MI. New drugs, called novel oral antiplatelet drugs (NOAPs), are more selective in targeting the various steps in the hemostasis/coagulation process. The combined therapeutic and adverse effects of these drugs are additive or synergistic.
MECHANISM OF ACTION
Platelets are essential participants in the blood coagulation process and in the formation of fibrin clots. By providing the initial hemostatic plug at the site of vascular injury, platelets prevent blood loss. But they also participate in the pathological thromboses that can lead to MI, stroke, and peripheral vascular thromboses when platelet activation is unwanted. Vessel injury—including physical damage to the vascular wall, destruction of the endothelium as a result of disease, and vascular wall irritation following stent placement—are conditions that may cause platelets to undergo adhesion to the vascular wall, activation, and aggregation. These platelet changes enhance activation of the blood coagulation process by providing a surface onto which clotting factors assemble and by releasing stored clotting factors.1
In a healthy vascular endothelium, several regulatory mechanisms, as well as numerous required co-factors work in concert to prevent initiation of the platelet activation and coagulation process.1 Upon vessel injury, platelets adhere to the vessel wall by interactions with von Willebrand factor and collagen, causing platelets to change shape and to release adenosine diphosphate (ADP). The activated platelets also generate thromboxane A2 (TxA2). Both ADP and TxA2 are agonists that cause further platelet activation and accumulation of platelets at the injury site. Disruption of the endothelial layer that serves as a barrier between circulating platelets and the prothrombotic subendothelial layer leads to exposure of tissue factor, which catalyzes the coagulation response. This response results in the formation of thrombin, which further activates platelets and encourages fibrinogen to form fibrin. The combination of activated platelets and fibrin at the injury site forms a stable hemostatic plug that arrests bleeding (Figure 1).2
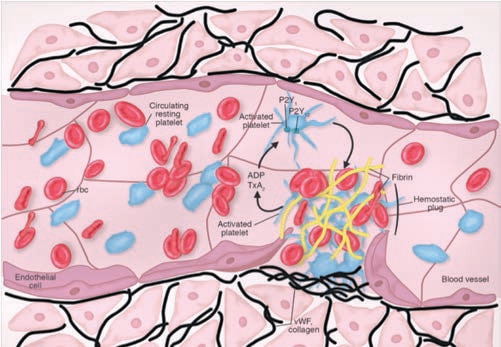
REPRINTED WITH PERMISSION FROM DORSAM RT, KUNAPULI SP. CENTRAL ROLE OF THE P2Y 12 RECEPTOR IN PLATELET ACTIVATION. J CLIN INVEST. 2004;113:340–345.
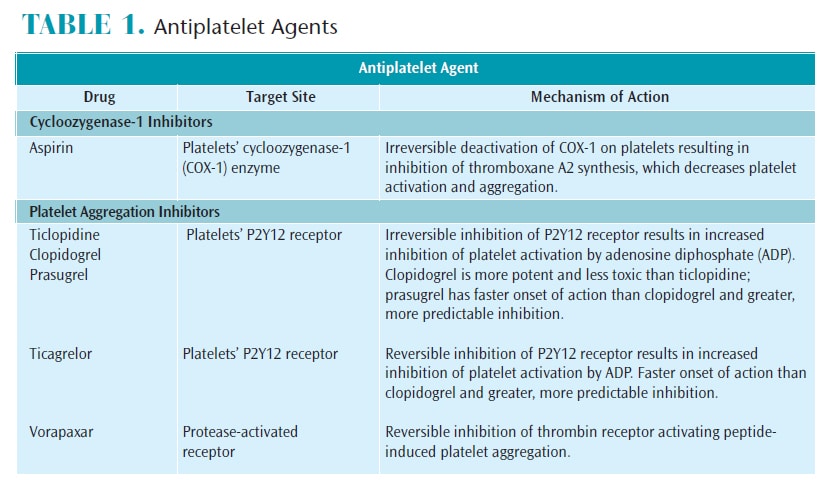
Platelets are key players in the blood coagulation cascade. Because platelets do not have nuclei, they do not have the ability to synthesize new proteins. Aspirin interferes with the production of TxA2 by blocking its synthesis from cyclooxygenase 1 (COX-1), thus, preventing platelet activation.1,3 Additionally, the activation of multiple receptors on the platelets’ surface leads to further platelet adhesion and aggregation. The ADP released following endothelial injury binds to purinergic receptors P2Y1 and P2Y12, leading to platelet activation. Reversible or irreversible inhibition of either purinergic receptors by several antiplatelet drugs is sufficient to block platelet activation (Table 1).1,2,4,5
These potent inhibitors of platelet function have revolutionized cardiovascular medicine and improved patient outcomes by lowering the risk of complications, such as restenosis and thrombosis.
ASPIRIN AND NOVEL ORAL ANTIPLATELETS
Aspirin is often used synergistically with other antiplatelet agents. Low-dose aspirin (75 mg to 81 mg), used alone or in combination with NOAPs, decreases the risk for MI events and stroke, and increases short- and long-term survival rates.6 Studies have shown that aspirin offers significant benefits in stroke prevention. Controversy remains on the preventive use of aspirin in low-risk patients when a risk of bleeding is present.7,8
Oral health professionals will likely encounter patients taking low-dose aspirin on a daily basis. Individuals on this regimen typically have a familial history of heart disease, previous MI and ischemic stroke, or type 2 diabetes.9 Patients taking aspirin without their physician’s recommendation should be advised to consult with their primary care providers.
NOAPs include thienopyridines (ticlopidine, clopidogrel, and prasugrel) and nonthienopyridines (ticagrelor and vorapaxar). Ticlopidine was the first thienopyridine introduced in 1991, followed by clopidogrel and prasugrel. Indications for ticlopidine’s use are primary and secondary prevention of stroke and dual antiplatelet therapy with aspirin for patients undergoing coronary stent placement.10 It may also be used in place of aspirin for those who are allergic.
All three drugs in this class are prodrugs, activated after partial metabolism in the body. They irreversibly inhibit the platelet P2Y12 receptors, preventing ADP from activating platelets and initiating their aggregation. Ticlopidine, clopidogrel, and prasugrel are known as ADP receptor antagonists.
Ticlopidine has several adverse effects, with the most serious being thrombotic thrombocytopenic purpura. This rare but potentially fatal platelet disorder is characterized by platelet thrombi and possible visible petechial and purpural lesions on the skin and mucosa. Development of aplastic anemia is another serious adverse effect. These hematologic effects have encouraged the more frequent use of its successor, clopidogrel.
Clopidogrel, introduced in 1997, is indicated for secondary prevention of stroke and MI, and for patients with peripheral artery disease and acute coronary syndrome.10–12 Clopidogrel is frequently prescribed with aspirin because it has a synergistic effect in reducing thrombosis after angioplasty and coronary stent placement, as well as prevention of ischemia in patients with unstable angina.1 Like ticlopidine, clopidogrel irreversibly inhibits P2Y12 receptors but is more potent and less likely to exert adverse hematologic effects.
Genetic variations in clopidogrel metabolism by hepatic drug metabolizers may prevent transformation to its active form. Clopidogrel now carries a warning issued by the United States Food and Drug Administration regarding this concern, as poor metabolism may result in significantly decreased effects.13,14 Like ticlopidine, clopidogrel is associated with thrombocytopenic purpura, though it is rare.15
Prasugrel is the newest P2Y12 platelet aggregation inhibitor. It is prescribed for the treatment of acute coronary syndrome, including unstable angina and nonST-elevation MI, or for patients with ST-elevation MI and for those who need percutaneous intervention (PCI) (Table 1). PCI, also known as coronary angioplasty, is used to open blocked coronary arteries and restore blood flow to the heart muscle. Prasugrel is converted to its activated form more easily than clopidogrel, and genetic metabolic resistance is not a concern. Prasugrel is contraindicated for individuals with a history of transient ischemic attack (TIA) or stroke, or for those older than 75 due to increased risk of intracranial bleeding.16
Prasugrel may cause significant and sometimes fatal bleeding, and it is the only medication in its class where medically supervised discontinuation prior to surgery may be indicated. However, managing the bleeding without discontinuation, if possible, is preferred.
NONTHIENOPYRIDINES
Ticagrelor and vorapaxar are nonthienopyridines. Ticagrelor also binds to the P2Y12 receptor but, unlike thienopyridines, it does not require partial metabolism to exert its effects. In addition, its platelet binding capabilities are reversible (Table 1).5 These two features enable ticagrelor to exert a more rapid onset of action and drug clearance and allow platelets to recover in 72 hours. With the previously described irreversible thienopyridines, platelets are replaced in 7 days to 10 days. Ticagrelor is most often prescribed twice daily with low-dose aspirin. It reduces the rate of cardiovascular death in patients with acute coronary syndrome and post MI, and reduces stent thrombosis.
Individuals taking ticagrelor must keep their aspirin dosage below 100 mg. Drug interactions may also be an issue, with certain agents prescribed in dentistry, such as some antibiotics and antiviral drugs, potentially impacting drug effectivenesss and posing a risk of gastrointestinal (GI) bleeding.15
Vorapaxar is unique among the NOAPs because it is a highly selective antagonist of a specific pathway of platelet aggregation: the protease-activated receptor-1 (PAR-1 or thrombin). Approved in 2014, vorapaxar works well in combination with aspirin and P2Y12 receptor antagonists to further reduce the risk of recurrent thrombosis, particularly recurrent MI.17 It is indicated for the prevention of cardiovascular thrombotic events for patients with a history of MI or peripheral artery disease. Vorapaxar is contraindicated for patients with a history of stroke, TIA, intracranial hemorrhage, and active pathological bleeding.
While platelet inhibition by vorapaxar is considered reversible, it has a very long half-life (3 days to 8 days). This makes it essentially irreversible and limits the effectiveness of short-term discontinuation, such as for planned surgical procedures. Vorapaxar should not be taken concurrently with drugs metabolized by the same enzymes. In dentistry, these may include some antivirals and antifungal drugs, as well as carbamazepine (sometimes prescribed for trigeminal neuralgia).
IMPLICATIONS FOR ORAL HEALTH CARE
The primary concern for patients taking antiplatelet agents in the dental setting is the possibility of an adverse bleeding event. Secondarily, drug-drug and drug-food interactions should be assessed. Current research and professional opinion discourage the discontinuation of oral antiplatelet medications for most dental procedures.18–21 Oral health professionals must consider patient characteristics and health history, extent of oral disease, concurrent medication use, and the type of dental procedure before consulting with the patient’s prescribing physicians regarding antiplatelet therapy and whether any changes are advised. Sudden discontinuation of these drugs increases the risk of thromboembolic events, such as stroke or MI. As such, any change or discontinuation of drug regimen, including aspirin, should be absolutely necessary and medically supervised. Therefore, any change in or discontinuation of drug regimen, including aspirin, should be done only when absolutely necessary and under medical supervision. A systematic review that focused solely on aspirin use in more than 50,000 subjects concluded discontinuation or noncompliance with aspirin therapy was associated with a high risk of major cardiac events, with patients with coronary stents at the greatest risk.22
Patients with recent coronary stent placement of less than 12 months should not prematurely discontinue dual antiplatelet therapy.23If possible, elective dental procedures should be postponed for 1 year. If postponement is not possible, aspirin therapy should be continued during the perioperative period to prevent stent thrombosis. Medical consultation is mandatory in these cases.
Less invasive procedures, such as scaling and root planing and simple extractions, will not cause enough bleeding to warrant discontinuation of antiplatelet medications.20,24 Patients on single and dual antiplatelet therapy may experience prolonged but not clinically significant bleeding following dental surgery.21 The use of a local anesthetic with a vasoconstrictor controls bleeding during the procedure and modulates systemic uptake of the adrenergic agent, and can safely be used for patients with cardiovascular disease at the recommended cardiac dose.25 Prolonged bleeding (lasting for several hours) after minimally invasive procedures can be managed with local hemostatic measures and should be reported to the prescribing physician for follow-up.21,26
Patients who take aspirin should avoid using nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen and naproxen. NSAIDs and aspirin competitively bind to COX-1 and COX-2 enzymes and inhibit prostaglandin, an enzyme that produces protective mucus in the lining of the GI tract. This combined GI irritation and antiplatelet effect may cause an increased risk of GI bleeding. Acetaminophen is a better choice for minor post-operative pain control in these circumstances.
Interactions caused by co-administration of antiplatelets with inhibitors or inducers of CYP450 enzymes vary. Prasugrel has negligible concerns and ticagrelor and vorapaxar may have significant interactions. Common drugs in this class include antibiotics such metronidazole, erythromycin, and clarithromycin, in addition to antifungals, including ketoconazole and fluconazole. These agents either induce or inhibit the desired effects of the antiplatelet drugs.
CONCLUSION
Antiplatelet drugs are increasing patient quality of life and lifespan. Assessment and elimination of oral infection and inflammation through prevention, education, and treatment are the first line of defense in controlling significant bleeding events in the dental setting. Remaining knowledgeable about cardiovascular diseases, pharmacotherapeutic agents used for their treatment and prevention, and risks that can contribute to problematic bleeding associated with oral procedures is important. Using current and evidence-based practice guidelines is essential when providing care to patients taking oral antiplatelet drugs.
REFERENCES
- Brunton LL, Chabner BA, Knollmann, Björn C. Goodman and Gilman’s The Pharmacological Basis of Therapeutics. 12th ed. New York: McGraw Hill; 2011.
- Dorsam RT, Kunapuli SP. Central role of the P2y12 receptor in platelet activation. J Clin Invest. 2004;113:340–345.
- Miner J, Hoffhines A. The discovery of aspirin’s antithrombotic effects. Tex Heart Inst J. 2007;34:179.
- Siller-Matula JM, Krumphuber J, Jilma B. Pharmacokinetic, pharmacodynamic and clinical profile of novel antiplatelet drugs targeting vascular diseases. Br J Pharmacol. 2010;159:502.
- Winter MP, Koziński M, Kubica J, Aradi D, Siller-Matula JM. Personalized antiplatelet therapy with P2Y12 receptor inhibitors: benefits and pitfalls. Postępy W Kardiologii Interwencyjnej Adv Interv Cardiol. 2015;11:259.
- Randomised trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2. ISIS-2 (Second International Study of Infarct Survival) Collaborative Group. Lancet. 1988;2:349–360.
- Ridker PM, Cook NR, Lee I-M, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;35:1293–1304.
- Luepker RV, Steffen LM, Duval S, Zantek ND, Zhou X, Hirsch AT. Population trends in aspirin use for cardiovascular disease prevention 1980–2009: the Minnesota Heart Survey. J Am Heart Assoc 2015;4:e002320.
- Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH, CLASSICS Investigators. Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting : the clopidogrel aspirin stent international cooperative study (CLASSICS). Circulation. 2000;102:624–629.
- Husted S. Antithrombotic therapy for long-term secondary prevention of acute coronary syndrome in high-risk patients. Ther Clin Risk Manag. 2015;11:263–277.
- Roe MT, Armstrong PW, Fox KAA, et al. Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367:1297–1309.
- United States Food and Drug Administration. FDA Drug Safety Communication: Reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drug. Available at: fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203888.htm. Accessed December 12, 2016.
- Qureshi Z, Hobson AR. Clopidogrel “resistance”: where are we now? Cardiovasc Ther. 2013;31:3–11.
- Zakarija A, Kwaan HC, Moake JL, et al. Ticlopidine- and clopidogrel-associated thrombotic thrombocytopenic purpura (TTP): review of clinical, laboratory, epidemiological, and pharmacovigilance findings (1989–2008). Kidney Int Suppl. 2009;112:S20–S24.
- Serebruany VL. Ticagrelor shift from PLATO to PEGASUS: Vanished mortality benefit, excess cancer deaths, massive discontinuations, and overshooting target events. Int J Cardiol. 2015;201:508–512.
- Toxnet Toxicology Data Network. Prasugrel. Available at: toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+7995. Accessed December 12, 2016.
- Morrow DA, Braunwald E, Bonaca MP, et al. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366:1404–1413.
- Bajkin BV, Vujkov SB, Milekic BR, Vuckovic BA. Risk factors for bleeding after oral surgery in patients who continued using oral anticoagulant therapy. J Am Dent Assoc. 2015;146:375–381.
- Becker DE. Antithrombotic drugs: Pharmacology and Implications for dental practice. Anesth Prog. 2013;60:72–80.
- Dézsi BB, Koritsánszky L, Braunitzer G, Hangyási DB, Dézsi CA. Prasugrel versus clopidogrel: a comparative examination of local bleeding after dental extraction in patients receiving dual antiplatelet therapy. J Oral Maxillofac Surg.2015;73:1894–1900.
- Johnston S. An evidence summary of the management of the care of patients taking novel oral antiplatelet drugs undergoing dental surgery. J Am Dent Assoc. 2016;14:271–277.
- Biondi-Zoccai GGL, Lotrionte M, Agostoni P, et al. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. Eur Heart J. 2006;27:2667–2674.
- Grines CL, Bonow RO, Casey Jr DE, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: A science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. J Am Dent Assoc. 2007;138:652–655.
- Lu SY, Tsai CY, Lin LH, Lu SN. Dental extraction without stopping single or dual antiplatelet therapy: results of a retrospective cohort study. Int J Oral Maxillofac Surg. 2016;45:1293–1298.
- Malamed SF. Handbook of Local Anesthesia. 6th ed. St. Louis: Mosby; 2013.
- Spolarich AE, Andrews L. An examination of the bleeding complications associated with herbal supplements, antiplatelet and anticoagulant medications. J Dent Hyg. 2007;81:67.
From Dimensions of Dental Hygiene. January 2017;15(1):34-37.



