
The Root of Dentinal Hypersensitivity
Clinicians are best prepared to effectively diagnose and manage this common problem through a comprehensive understanding of its etiology.
This course was published in the October 2014 issue and October 31, 2017. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Describe the theory on the pain mechanisms behind dentinal hypersensitivity.
- Explain the etiology and pathogenesis of sensitivity.
- Discuss the various methods of diagnosing dentinal hypersensitivity.
- Identify the different treatment options available.
INTRODUCTION
Dentinal hypersensitivity has been described as an enigma—frequently encountered, yet poorly understood. It is a condition that affects many patients—some rarely but others very frequently. Patients often encounter the symptoms, but don’t report their discomfort to their oral health professional. A multiplicity of causes is associated with hypersensitivity and it affects all types of patient populations. It can occur in older adults with gingival recession and exposed dentin, patients with poor brushing habits, and individuals with periodontal diseases. We encounter the condition in young patients as well, mainly due to enamel loss caused by tooth wear or erosion. Tooth wear at the cervical margin does not have to be extensive before underlying dentin is exposed and sensitivity ensues, but it is not easily noticed by clinicians.
Many patients manage their dentinal hypersensitivity by avoiding triggers, such as ice water and cold air. During a dental procedure, however, avoiding these stimuli is not possible. A large variety of products is available to help relieve the symptoms of sensitivity. Recent advances in technology have enabled the development of Colgate Pro-Argin® technology, which is based on arginine (an amino acid found in saliva) and calcium carbonate. The Pro-Argin technology is capable of blocking dentin tubules after application to the tooth surface with a prophy cup, thus helping patients alleviate the pain associated with dentinal hypersensitivity via a simple chairside procedure.
The Colgate-Palmolive Company is delighted to have provided an unrestricted educational grant to support this article in collaboration with the American Academy of Periodontology. I hope you find this article to be a valuable resource to help patients manage their sensitivity in your practice.
—Barbara Shearer, BDS, MDS, PhD
Director of Scientific Affairs
Colgate Oral Pharmaceuticals
FROM THE AMERICAN ACADEMY OF PERIODONTOLOGY
Periodontal diseases are a likely culprit in the development of dentinal hypersensitivity. As gingival tissue recedes and supportive bone wears away, exposed root surfaces make for an unsightly and unhealthy smile. Patients may also experience sharp pain when these roots—which lead to the tooth’s nerve center—come in contact with heat, cold, or pressure. The American Academy of Periodontology (AAP) supports the study of dentinal hypersensitivity and its contributory mechanisms in order to advance disease diagnosis and treatment. With the continuing education of all dental professionals, knowledgeable oral health teams will be prepared to provide the best possible care to patients.
In the third installment of this continuing education series, generously supported by Colgate-Palmolive, AAP member Sivaraman Prakasam, BDS, MSD, PhD, addresses the etiology and diagnosis of dentinal hypersensitivity, as well as possible treatment methods for the pain associated with this common oral problem. Prakasam contends that understanding the causes of dentinal hypersensitivity will help practitioners effectively manage each patient’s unique case.
—Joan Otomo-Corgel, DDS, MPH,
President, American Academy of Periodontology
Clinical Professor, Department of Periodontics,
University of California, Los Angeles, School of Dentistry
Dentinal hypersensitivity is typically a transient but sharp pain caused by thermal, chemical, tactile, evaporative, or osmotic stimuli.1 Historically, terms like cervical dentin sensitivity, dentin sensitivity, and root dentin hypersensitivity have been interchangeably used to describe this pain.2 The European Federation of Periodontology suggests that the term “root sensitivity” is best suited to specifically describe tooth sensitivity associated with periodontal diseases and their treatment.3 There is no evidence, however, for differentiation between coronal and radicular (dentin found in the anatomical root portion of a tooth) hypersensitivity in terms of etiology, diagnosis, or management.4
Hypersensitive dentin and normal dentin most likely have the same pain mechanisms.5 Several theories have been proposed to explain dentinal hypersensitivity.4 The most widely accepted is the hydrodynamic theory, which suggests that dentinal hypersensitivity arises when a stimulus causes fluid flow in the dentinal tubules, which then results in activation of pain receptors in the pulp/dentin border area.6 The displacement of fluid in the tubules is postulated to activate intradental myelinated A-? and some A-? fibers, resulting in the classical short, sharp pain of sensitivity. Human studies have shown a positive correlation between density of tubules in exposed dentin and pain responses to stimuli.7 Sensitive teeth have a greater number of tubules, which are twice as wide compared to nonsensitive teeth.8
 A number of stimuli can result in fluid displacement and evoke sensitivity. Air from the air-water syringe or cold, windy weather can result in surface desiccation of dentin and may cause outward flow of fluid in the dentinal tubules. Similarly, cold thermal stimulus or an osmotic stimuli can cause outward displacement of fluid in the tubules.9 Thermal, or hot, stimuli can result in contraction of fluid in the tubules. Physical stimuli may compress the dentin, with its subsequent release causing rebound expansion and increased outward fluid flow.10 Cold stimuli typically elicit the most intense sensitivity effects.11
A number of stimuli can result in fluid displacement and evoke sensitivity. Air from the air-water syringe or cold, windy weather can result in surface desiccation of dentin and may cause outward flow of fluid in the dentinal tubules. Similarly, cold thermal stimulus or an osmotic stimuli can cause outward displacement of fluid in the tubules.9 Thermal, or hot, stimuli can result in contraction of fluid in the tubules. Physical stimuli may compress the dentin, with its subsequent release causing rebound expansion and increased outward fluid flow.10 Cold stimuli typically elicit the most intense sensitivity effects.11
Fluid movement in dentinal tubules may cause an electrical streaming potential instead of or in addition to stimulating the nociceptors in the dentin/pulp border.12 It is not known whether these stimuli can agitate nerve fibers. Recent evidence proposes that odontoblasts may play a significant role in dentinal hypersensitivity. As odontoblasts are closely situated to nerve endings, their signals can be potentially transduced to the axons and vice versa. Odontoblasts express transient receptors that can potentially sense heat, cold, or fluid movements within the tubules, releasing mediators in the gap space between odontoblasts and axons.13
ETIOLOGY OF DENTIN HYPERSENSITIVITY
Addy14 described two essential steps for the occurrence of dentinal hypersensitivity: lesion localization and lesion initiation. In order for sensitivity to occur, the dentin must be exposed and the tubules opened. While a distinction is sometimes made between coronal and radicular sensitivity, there is no evidence to support differences in their pathogenesis. There are, however, several distinct structural differences between dentin of crown and dentin of root.4 Coronal dentinal tubules follow a double-curved course. Dentinal tubules beneath the incisal tips and in the root run much straighter. The number of tubules per unit area increases almost two-fold from the outer surface to the pulp. In addition, the radius of the tubules and the tubule lamina also increase as they approach the pulp. This results in a 20-fold increase in the wetness of dentin from superficial dentin to deep dentin when the tubules are patent.15 As such, deeper dentin may result in more sensitivity when compared to superficial dentin. Clinically, this is not always the case, which can be explained by the efficient formation of reactionary and reparative dentin that alters the patency of dentinal tubules. This is particularly true when exposure occurs gradually over time, as is in older adults.16 Rapid wear in young people can result in pain symptoms. Thus, the reparative capability, speed of the reparative process, rate at which dentin exposure occurs, and the age of the pulp can play significant roles in the etiology of dentinal hypersensitivity.
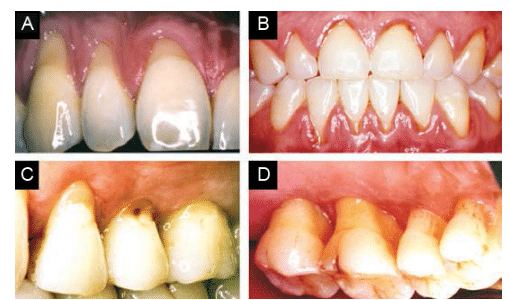
The exposure of dentin is caused either by the loss of soft tissue (Figure 1A and Figure 1B) or loss of hard tissue (Figure 1C and Figure 1D). Gingival recession is the most common etiology for exposure of root surface dentin (Table 1).14
Vigorous toothbrushing is a common cause of gingival recession in patients with healthy gingiva, particularly in individuals with obsessive toothbrushing habits (Figure 1A).4 Gingival biotype, thin alveolar bone (Figure 1A), factitious injuries, oral piercings, and past orthodontic treatment (Figure 1B) are other risk factors for gingival recession.4
Periodontal diseases and their treatment can result in an apical shift of soft tissue margin (Figure 1D) and loss of hard tissue surrounding the root.17 The amount of tissue loss depends on the type of therapy. Further loss of periodontal tissue can occur during the maintenance phase, depending on gingival biotype, patient compliance, and disease recurrence.18 The loss of tissue can occur at buccal, lingual, or interdental sites.19 Smoking is a risk factor for periodontitis and increases the risk of tissue loss depending on history of use and patient response to periodontal therapy.20
Periodontitis and its treatment can cause the loss of cementum, resulting in exposed radicular dentin (Figure 1D). Noncarious cervical lesions (Figure 1C) are some of the major etiological factors for dentin exposure at the gingival margin. These lesions occur more frequently in buccal and labial surfaces, but lingual and interproximal surfaces may also be affected.4 These lesions are often the result of abrasion, erosion, and abfractions.21 Physical wear caused by foreign substances or objects can lead to abrasive lesions at the gingival margin.22 Erosion is a result of chemical wear due to extrinsic acids, intrinsic acids, or chelators—such as gastric reflux, eating disorders, alcoholism, or pregnancy.22 Tensile or shear stress in the cementoenamel region can result in microfractures in enamel and dentin, leading to the development of abfractions.22
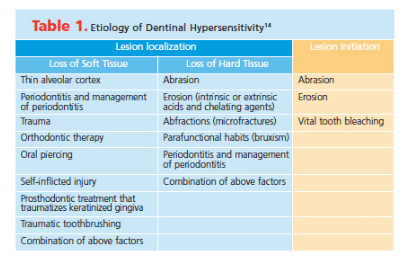 Dentin exposure can also occur naturally, as part of the developmental processes that establish the cementoenamel junction (CEJ). Muller and van Wyk23 demonstrated three types of hard tissue relationships in terms of the CEJ (Figure 2). They found that in 49% of the study population, the CEJ was in edge-to-edge contact; in 33% there was cementum overlap to enamel; and in 18% dentin was exposed for all teeth. Dentin exposure was particularly high in incisors/canines compared to premolars and molars.
Dentin exposure can also occur naturally, as part of the developmental processes that establish the cementoenamel junction (CEJ). Muller and van Wyk23 demonstrated three types of hard tissue relationships in terms of the CEJ (Figure 2). They found that in 49% of the study population, the CEJ was in edge-to-edge contact; in 33% there was cementum overlap to enamel; and in 18% dentin was exposed for all teeth. Dentin exposure was particularly high in incisors/canines compared to premolars and molars.
DIAGNOSIS
Dentinal hypersensitivity is a highly subjective condition and is often accompanied by other confounding problems. Gillam24 states that dentinal hypersensitivity is a diagnosis of exclusion. Due to the routine presence of confounding factors, a differential diagnosis must be established, which includes: reversible and irreversible pulpitis, cracked tooth syndrome, fractured restorations, fractured teeth, dental caries, periapical periodontitis, lateral periodontal abscess, periocoronitis, and, rarely, dry socket.24 The characteristics, duration, intensity, evoking stimuli, and relieving factors should be elicited as part of routine history taking. Extraoral and intraoral examinations must be conducted to find associated features, such as state of hard and soft tissue, swelling in the area, and systemic symptoms, which further aid in establishing the differential diagnosis.25
Various stimuli can be used to assess dentinal hypersensitivity. Mechanical or tactile stimuli can be applied with the help of a probe, during scaling procedures, or with a single tufted brush.24 Chemical stimuli can be applied with hypertonic solutions, such as sodium chloride, sucrose, glucose, and calcium chloride. Electrical pulp testers and dental pulp stethoscope can be used to create electrical stimulation. Evaporative stimuli can be applied through an air-water syringe and cold air blast. Thermal stimuli can be applied similarly to how the endodontic status off a tooth is determined. From a practical point of view, a tactile or thermal/evaporative stimuli may be used in conjunction with a visual analog scale (0 to 10) to record the severity of the patient’s response. Based on the diagnosis, severity of symptoms, and established etiology of dentinal hypersensitivity, a comprehensive management plan can then be customized for individual patients.
MANAGEMENT
Early identification of predisposing etiological factors is vital for the prevention of sensitivity. Once these factors are identified, preventive measures—including diet counseling, modification of oral hygiene techniques, correction of occlusal trauma, and discontinuation of parafunctional habits—can be instituted. The goal is to interfere with the pain mechanisms associated with the sensitivity, either transiently or permanently. The various therapeutic options available for treating sensitivity can be categorized into two major groups: therapies that impede or diminish neural transmission, or modalities that physically occlude the patent tubule, preventing fluid flow in the dentinal tubules.26 Potassium nitrate and some laser therapies target neural transmission, while most other treatments are aimed at preventing fluid flow from the dentinal tubules.
THERAPIES
Potassium nitrate is one of the few common therapeutic agents that targets the neural conductance, aiming to desensitize the nerve. Various over-the-counter desensitization toothpastes and at-home and in-office gels are available with potassium nitrate. Potassium nitrate’s mode of action is due to the increase in extracellular potassium ion concentration, which can depolarize the nerve and subsequently prevent it from repolarizing. In general, this is a safe treatment that does not harm the pulp. It has been shown to effectively desensitize affected teeth for up to 4 weeks with regular use.27 A majority of patients in one Japanese study reported relief from subjective symptoms throughout 12 weeks of examination.28 A 2006 meta-analysis, however, reported a lack of clear evidence to support the use of potassium nitrate toothpastes for the treatment of sensitivity.29
Several types of lasers have been proposed to help reduce symptoms of dentinal hypersensitivity. Low output diode lasers are thought to work by mediating an analgesic effect by impeding nerve transmission.30 Nd:YAG, a high output laser, is thought to work by blocking or contracting dentinal tubules and/or supporting analgesia.31 The CO2 laser is also thought to work by occluding or narrowing dentinal tubules.32 The mechanism of action for the low-output helium-neon laser is unknown and its effectiveness varies widely from 5% to 100%.33 Most studies conducted on lasers show that the modality is safe and will not induce thermal damage to the pulp.34 Evidence on the efficacy of lasers in managing dentinal hypersensitivity, however, is still emerging.
 Strontium salts occlude or partially occlude open tubules by precipitating insoluble metal compounds on tooth surface.35 Their effect on nerve depolarization may also relieve sensitivity symptoms.36 Several clinical studies conducted on dentifrices containing strontium salts show they provide subjective relief for patients.37,38 Dietary acids do not alter the occlusion of tubules, which results from the precipitation of strontium acetate;39 therefore, treatment with strontium salts may have some substantivity.
Strontium salts occlude or partially occlude open tubules by precipitating insoluble metal compounds on tooth surface.35 Their effect on nerve depolarization may also relieve sensitivity symptoms.36 Several clinical studies conducted on dentifrices containing strontium salts show they provide subjective relief for patients.37,38 Dietary acids do not alter the occlusion of tubules, which results from the precipitation of strontium acetate;39 therefore, treatment with strontium salts may have some substantivity.
Fluoride products occlude dentin tubules, providing relief from sensitivity.40 Varnish containing 5% sodium fluoride is effective for a 24-week period.41 The application of topical fluoride gel decreases post-operative sensitivity associated with tooth bleaching.42 Stannous fluoride, which has also been shown to be effective in addressing sensitivity,43 acts through precipitation on the dentin surface or by generating a high mineral content, which then blocks the tubules.5
Oxalates precipitate into dentinal tubules and block dentinal fluid flow, alleviating dentinal hypersensitivity.44 They are relatively insoluble in acid, thus potentially providing substantivity.45
Glutaraldehyde reacts with serum albumin in dentinal tubules, precipitating into them. This narrows the tubules and prevents fluid flow. Some studies report a reduction in sensitivity that ranges from 5% to 27%, which could be due to placebo effects.46 In Europe, there is a combination product of 5% glutaraldehyde and 35% hydroxyethyl methacrylate available that may be effective for up to 7 months to 9 months.46
Resins and adhesives are used to seal dentinal tubules to prevent fluid flow and consequent activation of nerve complex in close proximity to tubules and odontoblasts. Thin polymer-based materials, such as resins and dentin bonding agents, are applied over the dentin, which generates an artificial smear layer—sealing patent tubules.47 Dentin bonding agents have varying efficacy in desensitizing47 and are often used as a last resort.48
The formulation of arginine and calcium carbonate mimics the naturally occurring biological process of tubule occlusion by salivary glycoproteins49 and results in a plug, which renders the tooth resistant to acid and thermal attacks by reducing fluid flow movement. This technology49 contains a combination of arginine, bicarbonate (pH buffer), and calcium carbonate and helps in rapid desensitization.50 The benefit of a single treatment lasts for a minimum of 28 days.50 In the United States, arginine-calcium carbonate is available in an in-office prophylaxis paste. In Europe, the formulation is also available in dentifrice.
Amorphous calcium phosphate (ACP) is used to disseminate calcium and phosphate ions with the goal of enhancing tooth remineralization. It may occlude dentinal tubules, providing sensitivity relief.51 ACP is available in in-office applied gels, take-home whitening products, professionally dispensed gels, sealants, varnish, and over-the-counter toothpastes. More in vivo research is needed to support its role in sensitivity management.
Bioactive glass acts by precipitating a hydroxycarbonate apatite layer, thus, blocking patent tubules.52 Bioactive glass-based desensitizing agents are available only in-office prescribed dentifrices and in-office prophylaxis pastes. These products have been shown to be clinically more effective, in the short-term (6 weeks).52 Occlusion of tubules and reduction of hydraulic conductance with the use of bioglass has been demonstrated through in vitro experiments.53
Casein phosphopeptide (CPP)-ACP contains casein phosphopeptides that stabilize an amorphous form of calcium phosphate to sustain the calcium and phosphate ions, enabling their delivery into the tooth structure. It provides bioavailable calcium and phosphate that becomes available during acidic challenges, encouraging tooth remineralization. This process may aid in decreasing sensitivity, but the research does not show definitive efficacy.54 CPP-ACP is available in a professionally dispensed cream and gum.
Tri-calcium phosphate is a combination of beta tri-calcium phosphate and sodium lauryl sulfate that is designed to provide fluoride, phosphate, and calcium ions to the teeth, which may provide relief from sensitivity, although more research is necessary to prove its efficacy.55 It is available in a fluoride varnish and prescription toothpaste.
Root coverage in areas of gingival recession can help occlude patent dentinal tubules. Various options like pedicle graft, free gingival grafts, and allogeneic soft tissue grafts can be used to achieve root coverage. The amount of root coverage achieved depends on the presence/absence of interproximal bone. One recent systematic review noted a decrease in dentinal sensitivity after root coverage surgery was performed.56
CONCLUSION
Dentinal hypersensitivity is a debilitating condition that can affect quality of life. Patients often make behavioral changes, such as avoiding certain foods and postponing necessary dental care. Establishing a correct diagnosis along with considering differential diagnosis are key to managing this condition. Understanding the etiology can help dental professionals better plan and customize management of dentinal hypersensitivity. Addressing the root cause of sensitivity is paramount to preventing recurrences. Depending on the type of lesion localization, soft tissue and hard tissue augmentation procedures may be necessary. Any residual sensitivity can be managed with a variety of additional options.
ACKNOWLEDGEMENT
The author would like to thank Vanchit John, DDS, MSD, for some of the images used in this publication.
REFERENCES
- Canadian Advisory Board of Dentin Hypersensitivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc. 2003;69:221–216.
- Addy M. Dentine hypersensitivity: definition, prevalence, distribution and aetiology. In: Addy M, Embery G, Edgar WM, Orchardson R, eds. Tooth Wear and Sensitivity. London: Martin Dunitz, 2000:239-248.
- Sanz M, Addy M. Group D summary. J Clin Periodontol. 2002;29:195–196.
- West N, Lussi A, Seong J, Hellwig E. Dentin hypersensitivity: pain mechanisms and aetiology of exposed cervical dentin. Clin Oral Investig. 2013;17:9–19.
- Addy M, Dowell P. Dentine hypersensitivity—a review. J Clin Periodontol. 1983;10:351–363.
- Närhi M, Jyväsjärvi E, Virtanen A, Huopaniemi T, Ngassapa D, Hirvonen T. Role of intradental A-and C-type nerve fibres in dental pain mechanisms. Proc Finn Dent Soc. 1991;88:507–516.
- Narhi M, Kontturinarhi V. Sensitivity and surface condition of dentin—a sem-replica study. J Dent Res. 1994;73:42.
- Absi E, Addy M, Adams D. Dentine hypersensitivity. J Clin Periodontol. 1987;14:280–284.
- Matthews B, Vongsavan N. Interactions between neural and hydrodynamic mechanisms in dentine and pulp. Arch Oral Biol. 1994;39:S87–S95.
- Pashley DH. Mechanisms of dentin sensitivity. Dent Clin North Am. 1990;34:449–473.
- Kleinberg I, Kaufman H, Confessore F. Methods of measuring tooth hypersensitivity. Dent Clin North Am. 1990;34:515–529.
- Griffiths H, Morgan G, Williams K, Addy M. Dentine hypersensitivity: the measurement in vitro of streaming potentials with fluid flow across dentine and hydroxyapatite. J Periodontol Res. 1993;28:60–64.
- Magloire H, Maurin JC, Couble ML, et al. Topical review. Dental pain and odontoblasts: facts and hypotheses. J Orofac Pain. 2010;24:335.
- Addy M. Dentine hypersensitivity: new perspectives on an old problem. Int Dent J. 2002;52:367–375.
- Pashley D. Dynamics of the pulpo-dentin complex. Crit Rev Oral Biol Med. 1996;7:104–133.
- Smith AJ, Sloan AJ, Matthews JB, Murray PE, Lumley P. Reparative processes in dentine and pulp. In: Addy M, Embery G, Edgar WM, Orchardson R, eds. Tooth Wear and Sensitivity. London: Martin Dunitz; 2000:53–66.
- Velden U. Regeneration of the interdental soft tissues following denudation procedures. J Clin Periodontol. 1982;9:455–459.
- Rosling B, Serino G, Hellström MK, Socransky S, Lindhe J. Longitudinal periodontal tissue alterations during supportive therapy. J Clin Periodontol. 2001;28:241–249.
- Wennström JL, Serino G, Lindhe J, Eneroth L, Tollskog G. Periodontal conditions of adult regular dental care attendants. J Clin Periodontol. 1993;20:714–722.
- Ah B, Michele K, Johnson GK, Kaldahl WB, Patil KD, Kalkwart KL. The effect of smoking on the response to periodontal therapy. J Clin Periodontol. 1994;21:91–97.
- Mair LH. Wear in the mouth: the tribological dimension. Tooth Wear and Sensitivity. London: Martin Dunitz. 2000:181–188.
- Ganss C. Definition of erosion and links to tooth wear. Monogr Oral Sci. 2006;20:9–16.
- Muller CJ, van Wyk CW. The amelo-cemental junction. J Dent Assoc S Afr. 1984;39:799–803.
- Gillam DG. Current diagnosis of dentin hypersensitivity in the dental office: an overview. Clin Oral Investig. 2013;17:21–29.
- Aghabeigi B. Dental pain. In: Pain Research and Clinical Management. New York: Elsevier; 2002:181–190.
- Shiau HJ. Dentin hypersensitivity. J Evid Based Dent Pract. 2012;12:220–228.
- Tarbet WJ, Silverman G, Stolman JM, Fratarcangelo PA. Clinical evaluation of a new treatment for dentinal hypersensitivity. J Periodontol. 1980;51:535–540.
- Nagata T, Ishida H, Shinohara H, et al. Clinical evaluation of a potassium nitrate dentifrice for the treatment of dentinal hypersensitivity. J Clin Periodontol. 1994;21:217–221.
- Poulsen S, Errboe M, Lescay Mevil Y, Glenny AM. Potassium containing toothpastes for dentine hypersensitivity. Cochrane Database Syst Rev. 2006;3:CD001476.
- Wakabayashi H, Hamba M, Matsumoto K, Tachibana H. Effect of irradiation by semiconductor laser on responses evoked in trigeminal caudal neurons by tooth pulp stimulation. Lasers Surg Med. 1993;13:605–610.
- Liu HC, Lin CP, Lan WH. Sealing depth of Nd:YAG laser on human dentinal tubules. J Endod. 1997;23:691–693.
- Zhang C, Matsumoto K, Kimura Y, Harashima T, Takeda FH, Zhou H. Effects of CO2 laser in treatment of cervical dentinal hypersensitivity. J Endod. 1998;24:595–597.
- Kimura Y, Wilder-Smith P, Yonaga K, Matsumoto K. Treatment of dentine hypersensitivity by lasers: a review. J Clin Periodontol. 2000;27:715–721.
- Yilmaz HG, Cengiz E, Kurtulmus-Yilmaz S, Leblebicioglu B. Effectiveness of Er, Cr: YSGG laser on dentine hypersensitivity: a controlled clinical trial. J Clin Periodontol. 2011;38:341–346.
- Miller S, Truong T, Heu R, Stranick M, Bouchard D, Gaffar A. Recent advances in stannous fluoride technology: antibacterial efficacy and mechanism of action towards hypersensitivity. Int Dent J. 1994;44(Suppl 1):83–98.
- Markowitz K, Pashley D. Discovering new treatments for sensitive teeth: the long path from biology to therapy. J Oral Rehabil. 2008;35:300–315.
- Pearce NX, Addy M, Newcombe R. Dentine hypersensitivity: a clinical trial to compare 2 strontium densensitizing toothpastes with a conventional fluoride toothpaste. J Periodontol. 1994;65:113–119.
- West N, Addy M, Jackson R, Ridge D. Dentine hypersensitivity and the placebo response. J Clin Periodontol. 1997;24:209–215.
- Olley RC, Pilecki P, Hughes N, et al. An in situ study investigating dentine tubule occlusion of dentifrices following acid challenge. J Dent. 2012;40:585–593.
- Ehrligh J, Hochman N, Gedalia I, Tal M. Residual fluoride concentrations and scanning electron microscopic examination of root surfaces of human teeth after topical application of fluoride in vivo. J Dent Res. 1975;54:897–900.
- Ritter AV, Dias WdL, Miguez P, Caplan DJ, Swift Jr EJ. Treating cervical dentin hypersensitivity with fluoride varnish. J Am Dent Assoc. 2006;137:1013–1020.
- Armênio RV, Fitarelli F, Armênio MF, Demarco FF, Reis A, Loguercio AD. The effect of fluoride gel use on bleaching sensitivity: a double-blind randomized controlled clinical trial. J Am Dent Assoc. 2008;139:592–597.
- Miller JT, Shannon IL, Kilgore WG, Bookman JE. Use of a water-free stannous fluoride-containing gel in the control of dental hypersensitivity. J Periodontol. 1969;40:490–491.
- Cuenin MF, Scheidt MJ, O’Neal RB, et al. An in vivo study of dentin sensitivity: the relation of dentin sensitivity and the patency of dentin tubules. J Periodontol. 1991;62:668–673.
- Cunha-Cruz J, Stout J, Heaton L, Wataha J. Dentin hypersensitivity and oxalates a systematic review. J Dent Res. 2011;90:304–310.
- Kakaboura A, Rahiotis C, Thomaidis S, Doukoudakis S. Clinical effectiveness of two agents on the treatment of tooth cervical hypersensitivity. Am J Dent. 2005;18:291–295.
- Johnson G. Effects of various conditioners and cleaning agents on prepared dentin surfaces: a scanning electron microscopic investigation. J Prosthet Dent. 1974;31:422–430.
- Pol DG, Jonnala J, Chute M, Gunjikar T, Pol SD. Current strategy in the management of dentinal hypersensitivity. J Ind Dent Assoc. 2011;5:746.
- Kleinberg I. SensiStat. A new saliva-based composition for simple and effective treatment of dentinal sensitivity pain. Dent Today. 2002;21:42–47.
- Schiff T, Delgado E, Zhang Y, Cummins D, DeVizio W, Mateo L. Clinical evaluation of the efficacy of an in-office desensitizing paste containing 8% arginine and calcium carbonate in providing instant and lasting relief of dentin hypersensitivity. Am J Dent. 2009;22(Spec No A):8A–15A.
- Giniger M, MacDonald J, Ziemba S, Felix, H. The clinical performance of professionally dispensed bleaching gel with added amorphous calcium phosphate. J Am Dent Assoc. 2005;136:383–392.
- Du Min Q, Bian Z, Jiang H, et al. Clinical evaluation of a dentifrice containing calcium sodium phosphosilicate (Novamin) for the treatment of dentin hypersensitivity. Am J Dent. 2008;21:210–214.
- Wang Z, Sa Y, Sauro S, et al. Effect of desensitizing toothpastes on dentinal tubule occlusion: A dentine permeability measurement and SEM in vitro study. J Dent. 2010;38:400–410.
- Kowalczyk A, Botuli?ski B, Jaworska M, Kierklo A, Pawi?ska M, Dabrowska E. Evaluation of the product based on Recaldent technology in the treatment of dentin hypersensitivity. Adv Med Sci. 2006;51(Suppl 1):40–42.
- Karlinsey RL, Mackey AC. Solid-state preparation and dental application of an organically modified calcium phosphate. J Mater Sci. 2009;44:346–349.
- Chambrone L, Sukekava F, Araújo MG, Pustiglioni FE, Chambrone LA, Lima LA. Root-coverage procedures for the treatment of localized recession-type defects: a Cochrane systematic review. J Periodontol. 2010;81:452–478.
From Dimensions of Dental Hygiene. October 2014;12(10):55–62.



