
Subgingival Air Polishing
The use of glycine powder with this new technique may offer benefits to periodontal and implant maintenance therapy.
This course was published in the January 2014 issue and expires January 2017. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Define subgingival air polishing.
- List the types of air polishing powders available.
- Identify the correct technique for using subgingival air polishing.
- Discuss the precautions that should be observed when using subgingival glycine powder air polishing.
Subgingival air polishing is a relatively new procedure that uses a specially designed powder, which is safe to utilize on root surfaces and soft tissue, to remove biofilm from periodontal pockets.1–3 This powder can also be used to remove biofilm from other soft tissue niches in the oral cavity.4 The main objective of subgingival air polishing is to improve periodontal health by decreasing the inflammation and bleeding caused by the host response to bacteria in the periodontal pocket. When used correctly, subgingival air polishing is safe and effective in the removal of biofilm in both shallow and moderate to deep periodontal pockets.4 While it does not remove calculus, subgingival air polishing may reduce bleeding on probing and remove biofilm quickly without causing pain.5
THE TECHNOLOGY
Air polishing has been available as an alternative to using a prophy angle and rubber cup during supragingival polishing since the late 1970s. The technology uses a combination of abrasive particles with water and compressed air delivered through an air-polishing device. The most common abrasive agent used during supragingival air-polishing is a sodium bicarbonate powder that is specifically processed for use in an air-polishing device. Air polishing can remove plaque and stain while the tip, held 3 mm to 4 mm away from the enamel surface, is moved in a circular motion. Sodium bicarbonate powders, however, can cause damage to dentin, as well as composite and glass ionomer restorations.6–10
TYPES OF AIR-POLISHING POWDERS
Other air-polishing powders have been introduced as alternatives to sodium bicarbonate. Aluminum trihydroxide powder is available for patients who cannot tolerate sodium icarbonate, but its larger particle size is harder than sodium bicarbonate. While indicated for removal of heavy stain on enamel, aluminum trihydroxide powder can damage dentin, cementum, restorative materials, and dental implants.11 Calcium carbonate is another option for supragingival use. The abrasive particles are small and spherical, but they may cause defects on root surfaces.1
Air-polishing powder composed of bioactive calcium sodium phosphosilicate material (bioactive glass) may improve patient comfort and decrease sensitivity.12 The 50-micrometer (?m) particles fracture into smaller pieces upon impact with the enamel or dentin surface. Bioactive glass powder, however, may remove healthy dentin at a higher rate than it removes carious dentin,13 and it can strip enamel at half the volume of a tungsten carbide bur.14 More research is needed to support the safety of bioactive glass powder in air polishing.
CHOOSING THE RIGHT POWDER
The amount of abrasive in air-polishing powders is an important consideration when using this technique. Many factors determine the amount of surface damage caused by an abrasive agent. Particle size and hardness affect the result, as do other variables that occur when the powder is applied in clinical practice. Petersilka et al15 found that when powder is a constant, time had the greatest influence on defect depth—though powder and water settings and distance were all factors.
Distance from the surface and angulation of the spray can affect the size of the defect.16 Pelka et al1 demonstrated that treatment time and air-polishing device selection also impact the size of the defect due to differences in the focus of the spray.
SUBGINGIVAL AIR-POLISHING POWDERS
The abrasive nature of sodium bicarbonate, calcium carbonate, and bioactive glass contraindicate their safe use in subgingival air polishing. Two ingredients can be used safely in subgingival air-polishing powders: erythritol and glycine. The safety and efficacy of air-polishing powder containing erythritol, a sugar alcohol, are supported by a randomized clinical trial.17 In a 2013 study, Hagi et al17 found that patients who received air polishing with erythritol powder experienced less discomfort, decreased treatment times, and reduced bleeding on probing and probing depths compared to the control group treated with scaling and root planing. Particles of the erythritol powder measure only 14 ?m. While currently used in Europe, this powder is not yet available in the United States.18
A significant body of research supports the safety and efficacy of glycine powder in subgingival air polishing.19 In a study that compared the safety of glycine powder to sodium bicarbonate powder, the former was shown to be 80% less abrasive than conventional sodium bicarbonate powder on human root surfaces.20
Research has evaluated the effect of glycine powder air polishing (GPAP) on the gingiva compared to sodium bicarbonate powder and hand instrumentation. An animal-based study found that the use of an air-polishing device with sodium bicarbonate powder created gingival lesions, whose severity directly correlated with the amount of time the device was used on the gingiva.21 A small study conducted on human subjects found that those treated with sodium bicarbonate powder or hand instrumentation experienced more erosion of the gingival epithelium than those who received GPAP. After 14 days, the gingival epithelium was completely reestablished in all groups.22
SUBGINGIVAL GLYCINE POWDER AIR POLISHING
Research demonstrates that subgingival GPAP is effective at removing subgingival biofilm and cleaning root surfaces. In two studies, Petersilka et al2,3 looked at the technique’s ability to remove subgingival biofilm on the buccal and lingual surfaces of teeth and interproximal sites in patients with moderate to severe periodontal disease, as well as interproximal sites. Both studies included subjects who had completed initial therapy and were in a maintenance phase. Each study used a split-mouth design, in which two quadrants were assigned either subgingival GPAP for 5 seconds per site, or scaling and root planing with Gracey curets until plaque was no longer visible. In both studies, the sites that received the air polishing experienced significantly greater reduction in plaque than the sites treated with curets. The subjects in both studies cited GPAP as more comfortable than hand and power instrumentation.2,3
NEW DEVICE TO REACH DEEPER POCKETS
GPAP is also effective in deep pockets with the introduction of new device designs. Flemmig et al23 conducted a study on the effects of GPAP using a traditional device on subjects with severe periodontitis who had probing depths greater than 6 mm.
Patients were tested in a maintenance program, during which normal oral hygiene was performed for 3 months. The test group was thoroughly instrumented using hand and ultrasonic instrumentation 3 months prior to the maintenance visit. The patients were treated with GPAP for 5 seconds per tooth surface at the maintenance visit. Results showed that the greatest amounts of subgingival biofilm were removed in sites with probing depths of up to 4 mm.23 Because periodontal maintenance requires that biofilm be removed to the depth of a pocket—regardless of size—design innovations have been introduced that allow deeper penetration of the pocket (Figure 1A and Figure 1B).
The nozzles on the new air-polishing devices are designed for subgingival insertion so they can spray air-polishing powder through multiple openings on the tip in order to remove biofilm from the root surface and the soft tissue lining the sulcus. The nozzle is inserted directly into the sulcus. Tip thickness is similar to that of most periodontal probes, with the wider dimension inserting parallel to the root surface and slipping easily into the pocket. The glycine powder air polisher nozzles have multiple outlets located above the tip that direct the glycine powder toward the root and epithelium of the pocket.

Moene et al5 conducted a study in 2010 evaluating patient acceptance, safety, and post-treatment biofilm levels with one of these subgingival GPAP devices. Subjects reported that the GPAP was more comfortable and less painful than hand instrumentation. In addition, the mean time required by clinicians for use of the subgingival glycine powder air polisher was significantly less than what was needed to instrument the sites with curets (0.5 minutes per site vs 1.4 minutes per site). No significant difference between microbiological levels was reported.5
ORAL SOFT TISSUE BACTERIAL REMOVAL
Biofilm has many niches in the oral cavity, including but not limited to supra- and subgingival surfaces on teeth. Bacterial species are also found on buccal and labial mucosa, the floor of mouth, soft palate, and tongue. While dental hygienists often spend significant time cleaning tooth surfaces, the deep crevices on the tongue that are teeming with biofilm may be missed. Flemmig et al4 found that subgingival GPAP helped reduce the bacterial load on the oral mucosa and tongue. In the study, one group received subgingival GPAP in moderate to deep pockets, as well as conventional air polishing on the oral mucosa and tongue. The control group was treated with scaling and root planing with manual instruments, followed by coronal polishing with a rubber cup and polishing paste. The results showed the group that received the air polishing had significantly lower bacterial counts in the moderate to deep pockets at baseline, as well as 10 days post-treatment. At 90 days, the test group had significantly lower Porphyromonas gingivalis counts in the oral cavity. There were no adverse events, and comfort levels were rated high in the air polishing group.4
AIR POLISHING vs ULTRASONIC DEBRIDEMENT
While many clinical trials compare new treatments to the gold standard of hand-instrumentation, many dental hygienists today are using ultrasonic scalers in their practice. Therefore, it is important to examine the literature that makes a direct comparison of microbiological outcomes between the use of subgingival GPAP and ultrasonic instrumentation during maintenance therapy. As stated previously, subgingival GPAP does not remove calculus; therefore, it does not replace mechanical debridement of calculus through power or hand instrumentation. It may, however, affect microbial parameters and bleeding on probing.
A study comparing subgingival GPAP for 5 seconds per site at 75% water and power to ultrasonic instrumentation for 30 seconds per site at 75% power on patients with moderate to deep periodontal pockets with bleeding on probing was conducted. Significant reduction in bleeding on probing and periodontal pocket depth, as well as bacterial species—both immediately and 2 days after treatment—was noted.24
No statistically significant differences were observed in clinical or microbiological variables between the two groups. The GPAP group, however, was completed in 10 seconds per site compared to 30 seconds per site in the ultrasonic debridement group, with the shorter duration also being judged by subjects as the most comfortable.24
TREATMENT OF PERI-IMPLANT DISEASES
Despite the remarkably high success rate of dental implant therapy, increasing numbers of patients are developing peri-implant mucositis or peri-implantitis—both of which are infectious diseases.25 A recent systematic review and meta-analysis reported a frequency of peri-implant mucositis among 63.4% of implant patients and 30.7% of implants, and a prevalence of peri-implantitis among 18.8% of patients and 9.6% of implants after at least 5 years of function.26 Klinge et al27 suggest that approximately one in five patients will experience peri-implantitis.
Subgingival GPAP can be a helpful addition to clinicians’ efforts to prevent both peri-implant mucositis and peri-implantitis. Experts at the 2012 Consensus Conference of the European Association for Osseointegration concluded that peri-implant mucositis can be successfully treated nonsurgically, and that all treatment modalities should disrupt the submucosal biofilm.27 A systematic review of nonsurgical treatment for peri-implantitis found that submucosal use of subgingival GPAP was more effective in reducing bleeding on probing than hand instrumentation with curets, in addition to adjunctive chlorhexidine irrigation.28
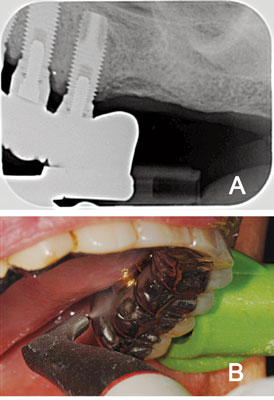
Subgingival GPAP is highly efficient in removing biofilm from the implant surface and pocket area, and also under difficult-to-access areas of implant-supported dentures (Figure 2A and Figure 2B). A recent in vitro study compared the effects—specifically surface roughness and cleaning effectiveness—of 10 types of cleaning instruments on bacterial cultures formed on titanium disks, which simulated implant surfaces.29 One of the instruments used was an air-polishing device with glycine powder particles of less than 63 ?m. The disks that received air polishing earned the best total score for cleanliness and also caused the least amount of damage compared to the other instruments/ devices tested, which included traditional prophylaxis, plastic curets, and an erbium laser.29
TECHNIQUE
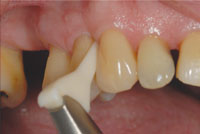
Several basic technique points should be followed when utilizing subgingival GPAP. To minimize the spread of dental aerosols and air-polishing powder, high-volume evacuation should be used when using an air polisher. Glycine powder can be used in most commercially available air-polishing devices; however, in order to reach up to 10 mm in a pocket, a subgingival nozzle that can be placed into the sulcus is required. The nozzle is inserted into the pocket gently until resistance is felt, then moved slightly back from the base of the pocket to allow for at least a 3 mm distance from the bone. The tip is then activated and moved over the entire subgingival root surface using a circular motion for 5 seconds per surface (Figure 3).
The thoughtful selection of air-polishing device, technique, powder, and nozzle will improve outcomes and help minimize damage to root surfaces. Patients who present with deep pockets will benefit from GPAP with a subgingival nozzle, which reaches farther and creates little damage to the tooth surface.
PRECAUTIONS
A traditional air-polishing device should never be pointed directly into the gingival sulcus or periodontal pockets due to the risk of iatrogenic facial emphysema—the holding of air in subcutaneous tissues.30 Subgingival GPAP uses a special application tip that is designed for subgingival use. The risk of facial emphysema does exist with this technique, but it is low. There have been three incidents of facial emphysema resulting from the use of subgingival GPAP reported in the literature. Each case was resolved within a couple of days without additional intervention.17 During the clinical trials performed on GPAP, no cases of facial emphysema were reported.2–5,19,23,24
Flemmig et al4 estimate that the probability of facial emphysema resulting from GPAP is approximately one in 666,666. Symptoms of facial emphysema caused by dental treatment include facial swelling, tenderness, and pain.30–32 The condition requires immediate treatment to prevent long-term, significant health consequences. Emergency health care providers may be unfamiliar with facial emphysema, so dental professionals must be able to detect the symptoms quickly.30 While the potential of facial emphysema exists with any device that uses air pressure, the risk appears to be low.33 Clinicians need to exercise caution when utilizing such devices, however, and explicitly follow all manufacturer instructions.
CONCLUSION
Clinicians should be mindful that calculus removal and biofilm removal are two distinct procedures. Subgingival GPAP is not intended to remove calculus—rather it complements power or hand instrumentation in the ultimate goal of eliminating biofilm. This new technique is supported by scientific evidence and warrants further exploration by clinicians. With a growing prevalence of peri-implantitis and peri-mucositis, subgingival GPAP has significant benefits to offer as a routine part of periodontal and implant maintenance therapy.
REFERENCES
- Pelka M, Trautmann S, Petschelt A, LohbauerU. Influence of air-polishing devices andabrasives on root dentin-An in vitro confocal laser scanning microscope study. Quintessence Int. 2010;41:e141–148.
- Petersilka GJ, Steinmann D, Haberlein I,Heinecke A, Flemmig TF. Subgingival plaque removal in buccal and lingual sites using a novel low abrasive air-polishing powder. J Clin Periodontol. 2003;30:328–333.
- Petersilka GJ, Tunkel J, Barakos K, Heinecke A,Haberlein I, Flemmig TF. Subgingival plaque removal at interdental sites using a low-abrasive air polishing powder. J Periodontol. 2003;74:307–311.
- Flemmig TF, Arushanov D, Daubert D, RothenM, Mueller G, Leroux BG. Randomized controlled trial assessing efficacy and safety of glycine powder air polishing in moderate-to-deep periodontal pockets. J Periodontol. 2012;83:444–452.
- Moene R, Decaillet F, Andersen E, Mombelli A. Subgingival plaque removal using a new air polishing device. J Periodontol. 2010;81:79–88.
- Galloway SE, Pashley DH. Rate of removal of root structure by the use of the Prophy-Jet device. J Periodontol. 1987;58:464–469.
- Yap AUJ, Wu SS, Chelvan S, Tan ES. Effect of hygiene maintenance procedures on surface roughness of composite restoratives. Oper Dent.2005;30:99–104.
- Wu SS, Yap AUJ, Chelvan S, Tan ESF. Effect of prophylaxis regimens on surface roughness of glass lonomer cements. Oper Dent.2005;30:180–184.
- Pelka MA, Altmaier K, Petschelt A, Lohbauer U.The effect of air-polishing abrasives on wear of direct restoration materials and sealants. J Am Dent Assoc. 2010;141:63-70.
- Graumann SJ, Sensat ML, Stoltenberg J. Airpolishing: a review of current literature. J Dent Hyg. 2013;87:173–178.
- Johnson WW, Barnes CM, Covey DA, WalkerMP, Ross JA. The effects of a commercial aluminum air polishing powder on dental restorative materials. J Prosthodont.2004:13;166–172.
- Banerjee A, Hajatdoost-Sani M, Farrell S,Thompson I. A clinical evaluation and comparison of bioactive glass and sodium bicarbonate air-polishing powders. J Dent.2010;38:475–479.
- Paolinelis G, Banerjee A, Watson TF. An in vitro investigation of the effect and retention of bioactive glass air-abrasive on sound and carious dentine. J Dent. 2008;36:214–218.
- Banerjee A, Paolinelis G, Socker M, McDonaldF, Watson TF. An in vitro investigation of the effectiveness of bioactive glass air-abrasion in the‘selective’ removal of orthodontic resin adhesive. Eur J Oral Sci. 2008;116:488–492.
- Petersilka GJ, Bell M, Mehl A, Hickel R, Flemmig TF. Root defects following air polishing.J Clin Periodontol. 2003;30:165–170.
- Tada K, Wiroj S, Inatomi M, Sato S. Thecharacterization of dentin defects produced by air polishing. Odontology. 2012;100:41–46.
- Hagi TT, Hofmanner P, Salvi GE, Ramseier C,Sculean A. Clinical outcomes following sub -gingival application of a novel erythritol powderby means of air polishing in supportive periodontal therapy: a randomized, controlled clinical study. Quintessence Int. 2013;44:753–761.
- Davis K. Biofilm removal with air polishing and subgingival air polishing. Available at: www.ineedce.com/courses/2507/PDF/1309cei_davis_web.pdf. Accessed December 2, 2013.
- Giacomelli L, Salerno M, Derchi G, GenovesiA, Paganin PP, Covani U. Effect of air polishing with glycine and bicarbonate powders on a nanocomposite used in dental restorations: anin vitro study. Int J Periodontics Restorative Dent. 2011;31:e51–56.
- Petersilka GJ, Bell M, Haberlein I, Mehl A,Hickel R, Flemmig TF. in vitro evaluation of novel low abrasive air polishing powders. J Clin Periodontol. 2003;30:9–13.
- Kozlovsky A, Artzi Z, Nemcovsky CE,Hirshberg A. Effect of air-polishing devices on the gingiva: histologic study in the canine. J Clin Periodontol. 2005;32:329–334.
- Petersilka G, Faggion CM Jr, Stratmann U, etal. Effect of glycine powder air-polishing on the gingiva. J Clin Periodontol. 2008;35:324–332.
- Flemmig TF, Hetzel M, Topoll H, Gerss J,Haeberlein I, Petersilka G. Subgingival debridement efficacy of glycine powder airpolishing. J Periodontol. 2007;78:1002–1010.
- Wennstrom JL, Dahlen G, Ramberg P.Subgingival debridement of periodontal pockets by air polishing in comparison with ultrasonic instrumentation during maintenance therapy. J Clin Periodontol. 2011;38:820–827.
- Lindhe J, Meyle J, Group D of European Workshop on Periodontology. Peri-implant diseases: Consensus Report of the SixthEuropean Workshop on Periodontology. J ClinPeriodontol. 2008;35(8 Suppl):282–285.
- Atieh MA, Alsabeeha NH, Faggion CM Jr,Duncan WJ. The frequency of peri-implant diseases: a systematic review and meta-analysis.J Periodontol. 2013;84:1586–1598.
- Klinge B, Meyle J, Working Group 2. Periimplant tissue destruction. The Third EAOConsensus Conference 2012. Clin Oral ImplantsRes. 2012;23(6 Suppl):108–110.
- Muthukuru M, Zainvi A, Esplugues EO,Flemmig TF. Non-surgical therapy for the management of peri-implantitis: a systematic review. Clin Oral Implants Res. 2012;23(6Suppl):77–83.
- Schmage P, Thielemann J, Nergiz I, ScorzielloTM, Pfeiffer P. Effects of 10 cleaning instruments on four different implant surfaces. Int J Oral Maxillofac Implants. 2012;27:308–317.
- Barnes C. Air polishing: a mainstay for dental hygiene. Available at: www.ineedce.com/courses/2423/PDF/1305cei_Barnes_RDH_final.pdf.Accessed December 4, 2014.
- Petersilka G. Air emphysema in periodontal therapy: a case series and critical literature overview (in German). Parodontologie. 2010:165–175.
- Frü?hauf J, Weinke R, Pilger U, Kerl H,Müllegger RR. Soft tissue cervico facial emphysema after dental treatment: report of 2 cases with emphasis on the differentialdiagnosis of angioedema. Arch Dermatol. 2005;141:1437–1440.
- Tan WK. Sudden facial swelling:subcutaneous facial emphysema secondary to use of air/water syringe during dental extraction.Singapore Dent J. 2000;23(1 Suppl):42–44.
From Dimensions of Dental Hygiene. December 2013;11(12):69–73.



