LIGHTFIELDSTUDIOS/ISTOCK/GETTY IMAGES PLUS
LIGHTFIELDSTUDIOS/ISTOCK/GETTY IMAGES PLUS
Sensitivity Management After Nonsurgical Periodontal Therapy
Accurate diagnosis and implementation of evidence-based therapies are key to addressing this common patient complaint.
Tooth sensitivity is a common complaint, especially following periodontal debridement, a key component of nonsurgical periodontal therapy (NSPT). Instrumentation during NSPT may inadvertently lead to exposed dentin, open dentinal tubules, and root sensitivity. This article will discuss the diagnosis and treatment of sensitivity following NSPT.
Dentinal or root sensitivity is defined as brief, sharp pain arising from exposed dentin when stimulated by temperature change, touch, pressure, or chemical means unrelated to other tooth-related defects or pathology.1 Sensitivity begins with the loss of hard tissue, enamel, or cementum attached directly or partially to the dentin layer of a tooth, which is called “lesion localization.”2 Several open or unobstructed dentinal tubules close together that create a pathway from the oral environment to the tooth pulp is termed “lesion initiation.” Both lesion localization and initiation must be present for an individual to feel pain.3 During NSPT, hard tissues are cut or planed with hand or ultrasonic instruments. During instrumentation, a thin, natural protective smear layer forms as a by-product. The 1-micron- to 2-micron-thick smear layer initially embeds at different depths into open dentinal tubules, reducing the permeability and sensitivity of the underlying dentin. However, this smear layer is easily altered by changes within the oral environment, such as variations in acidity.4
The hydrodynamic theory is the most widely accepted explanation for sensitivity due to exposed dentinal tubules lacking a protective smear layer. Various stimuli within the oral environment alter the flow and pressure of fluid within dentinal tubules, allowing mechanoreceptors surrounding odontoblastic processes—which occupy much of the space within a dentinal tubule—to detect these changes via hydrodynamic action.4,5 Odontoblasts act as receptor cells that mediate changes occurring within synaptic junctions inside the nerve.1,5 As this pressure change approaches the junction of the dentin and the pulp, sensory neurons called nociceptors are activated, stimulating electrical signals which relay the pain response to the brain. Three different types of nerve pain fibers are located on the pulpal side of the dentinal tubule that induce a variety of pain response to stimuli (eg, rapid, sharp, dull, aching).4
The combination of altered dentinal surfaces and the compromised smear layer from changes within the oral environment may result in an increase in dentin permeability, bacterial invasion of tubules, and/or pulpal irritation following NSPT. Tooth erosion, abrasion, attrition, gingival recession, cracked tooth, or anatomical defects are other alterations to tooth structure that may contribute to sensitivity beyond those caused by NSPT.6
PREVALENCE
Sensitivity typically affects adults between the ages of 30 and 60. Approximately 63% to 90% of patients experience sensitivity following NSPT. At 1 week post-NSPT, the number drops to 53% to 55% of patients still reporting sensitivity.2,7,8 Natural mechanisms of the body’s own healing, repair, and subsequent decrease in or cessation of sensitivity often occurs within 2 weeks following NSPT.9 During NSPT appointments, oral health professionals need to inform patients of this potential for short- or long-term sensitivity and emphasize the importance of thorough biofilm removal and use of fluoride dentifrice.10 For sensitivity that does not subside naturally within 2 weeks, removal of stimuli and the implementation of products designed to block or interrupt pulpal nerve stimulus are indicated.3 Patients should be advised to contact their oral health professional if sensitivity stops improving or worsens after several weeks. If a patient has persistent sensitivity, he or she should seek clinical evaluation so other potential diagnoses can be ruled out and an assessment of sensitivity severity to at least two stimuli can be conducted.11
DIAGNOSIS
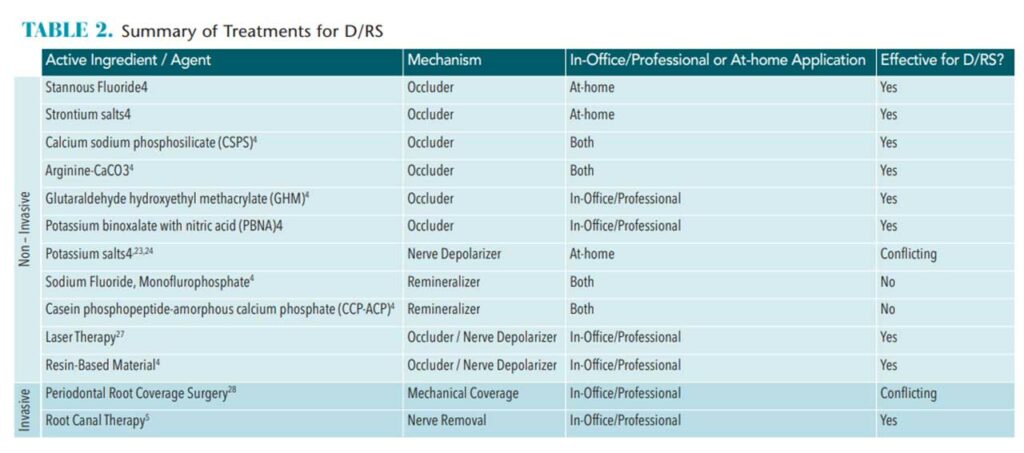
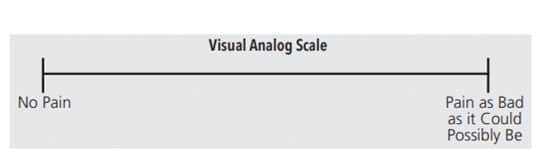
Sensitivity following NSPT requires an accurate diagnosis to ensure the most appropriate course of treatment is implemented. Tactile sensitivity tests, such as the Yeaple probe test, and cold air sensitivity tests (eg, Schiff scale) are common methods to confirm and assess the severity of sensitivity.3,4 These tests use instruments to apply pressure to the sensitive area and then the patient reports when the pain begins.12 A visual analog scale (VAS) is used to quantify responses for an assessment and can be used in conjunction with a tactile sensitivity test. The VAS contains a 10 cm long line, indicating the perceived level of pain experienced by the patient (Figure 1). Some VASs include a variety of face emojis that enable the patient to clearly identify his or her pain level (Figure 2,).11 The patient is asked to mark on the line or select the appropriate emoji that corresponds to his or her pain level.13 With the cold air sensitivity tests, the operator applies a brief puff of air with an air/water syringe to the affected tooth or site. The patient’s sensitivity is then rated on a scale from 0 to 3:
- 0 if the patient does not respond to air stimulus.
- 1 if the patient responds to air stimulus, but does not ask clinician to stop blowing air.
- 2 if the patient responds to air stimulus and requests to stop blowing air or moves away.
- 3 if the patient moves away or considers the air painful and requests the clinician to stop blowing air.5
Based on patient history, timing, extent, severity, responses to various stimuli, and ruling out other potential contributory factors, a diagnosis of sensitivity can be made. The clinician can then begin to identify the appropriate treatment recommendations. Treatments for sensitivity include agents that reduce permeability of dentinal tubules (occlude, restore), decrease neuronal response (depolarize nerve), and remineralize lost tooth structure (change composition). Laser therapy to occlude tubules and/or depolarize nerve is another option. More invasive procedures include periodontal surgery (mechanical coverage of exposed tooth surfaces) and root canal therapy (pulpal removal).4 Ideal sensitivity treatments should be easily administered, effective, safe, fast-acting, and provide a long-lasting effect.14 Following is a discussion of evidence-based treatments for tooth sensitivity.
OVER-THE-COUNTER OCCLUDING AGENTS
Occluding agents use a chemical precipitation of minerals and/or proteins to seal off surface openings of dentinal tubules and prevent hydrodynamic disruption of the fluid inside. Many dentifrices contain occluding agents, and these tend to be well-received by patients and are cost effective. Active ingredients used to occlude dentinal tubules in over-the-counter dentifrices include strontium salts, stannous fluoride, calcium sodium phosphosilicate (CSPS), and arginine with calcium carbonate (arginine-CaCO3).3
Strontium salts seal the tubular surface to prevent fluid flow fluctuations within the dentinal tubule to reduce stimulation of the nerve and thus sensitivity.3
Stannous fluoride deposits on mineralized tooth surfaces mimic the natural smear layer of insoluble metal salts covering dentinal tubules. This reduces dentin permeability and prevents fluid flow change and nerve stimulation.3 In the past, stannous fluoride caused extrinsic tooth stain but today’s stannous fluoride products include stabilizers, such as chelants, polyphosphates, and silicas, that have eliminated the staining side effect.6,15
CSPS produces a layer of hydroxyapatite to block the dentinal tubules, preventing fluid flow change and nerve stimulation.3,16
Arginine-CaCO3 works to raise the pH, creating a more basic oral environment, in which calcium phosphate salts then precipitate from saliva and gingival crevicular fluid. The precipitate occludes dentinal tubules and reduces fluid flow change and nerve stimulation.17
As with many patient-administered products, poor compliance with instructions can interfere with efficacy. For example, dispensing the improper dosage or amount, applying improperly, and using for less than the recommended time all impede effectiveness, making patient education key.16 The method of application for occluding at-home dentifrices may vary by brand but most suggest brushing with the prescribed amount of product for 2 minutes twice per day.6
IN-OFFICE OCCLUDING AGENTS
CSPS and arginine-CaCO3 are also available in prophylaxis paste. Traditional prophy pastes are used for polishing, but when desensitizing agents are added, they can also provide therapeutic benefits. The paste should be fine grit, applied with a slow speed handpiece at a rate of 1,500 rpm to 3,000 rpm, and with enough pressure for the rubber cup to flare onto interproximal surfaces.6 The clinician should begin at the gingival third and transition up to the incisal or occlusal third of each affected tooth/surface.6 When using prophy paste to treat sensitivity, the paste should only be applied to areas of sensitivity.
Other options for in-office desensitizing occluders are gels or varnish containing glutaraldehyde hydroxyethyl methacrylate (GHM) or potassium binoxalate with nitric acid (PBNA). These are often used during tooth preparation prior to placement of a restoration; however, they may also be used to address sensitive areas following NSPT. Gels or varnish containing GHM penetrate deep into the dentinal tubules and create several layers of proteins, which block the fluid from flowing toward and stimulating the nerve. The application of GHM includes cleaning the sensitive area, rinsing, isolating, applying the gel with a microbrush for 30 seconds to 60 seconds, drying thoroughly, and then rinsing again with water.
Gels containing PBNA work by creating calcium oxalate and potassium nitrate micro-crystals that penetrate into and seal the dentinal tubules, preventing fluid from entering. The initial application of PBNA may occur in a wet or dry environment, then should be scrubbed or burnished into the sensitive area with a microbrush for 20 seconds, followed by drying for 30 seconds.
The multistep application processes for GHM and PBNA may be repeated several times during the same appointment until the VAS response to a tactile sensitivity test is 1, or until the patient does not respond to the air stimulus.
NERVE DEPOLARIZING AGENTS
Evidence suggests that chemical agents, such as those in dentifrice, can reduce sensitivity by affecting pain conduction within the nerve.3 Pain occurs when a pressure change approaches the odontoblastic junction of the dentin tubule and the pulp, stimulating the pain response from the nerve to the brain.10 The mechanism of action for nerve depolarizing agents increases potassium concentration adjacent to the nerve. If potassium ions remain in the dentinal tubules and avoid transferring into the nerve cell, the cell cannot depolarize (or respond to pain stimuli). This blocks transmission of the pain response, thus reducing sensitivity.18 Nerve depolarizing agents containing potassium salts interrupt the pain response by providing external excess potassium in the local area of the dentinal tubules. At-home nerve depolarizing dentifrices contain potassium nitrate, potassium chloride, or potassium citrate with a concentration of ≥ 2% potassium ion.18 The method of application for these toothpastes is standard (2 minutes, twice daily).6
Evidence on the effectiveness of potassium salts in reducing sensitivity is mixed. Systematic reviews from 2006 and 2012 did not find much evidence supporting their efficacy; however, a more recent 2019 systematic review did find evidence that potassium salts reduce sensitivity.3,19,20 This discrepancy may be due to the timeframe of studies or methods for determining reductions in sensitivity.20
REMINERALIZING AGENTS
Demineralization is the loss of calcium and phosphate from the hydroxyapatite in the enamel. Over time, this loss of structure allows access to dentinal tubules, odontoblasts, or pulpal areas of the tooth, thus stimulating nerve fibers and causing pain. Remineralization is the process of repairing the lost tooth structure through the use of fluoride.6 The enamel structure changes from hydroxyapatite to fluorapatite as fluoride is absorbed into this demineralized area of tooth structure. This new surface allows remineralization to occur more rapidly than on the original hydroxyapatite structure; is more resistant to further demineralization from pH; and may decrease sensitivity caused by temperature, bacteria, or air. Topical fluoride delivery is available via low-concentration or high-concentration dentifrice, mouthrinses, foams, prophy paste, and varnishes.6 Sodium fluoride and monofluorophosphate are highly effective remineralization agents that replace lost enamel minerals; however, there is insufficient evidence to demonstrate their effectiveness in reducing sensitivity after NSPT.3 Some fluoride-containing remineralizing agents contain calcium compounds that enhance delivery of calcium and phosphates, creating a precipitate, which can facilitate occlusion of the dentinal tubules.6 Casein phosphopeptide-amorphous calcium phosphate (CCP-ACP) is in-office agent that comes as a prophy paste or varnish. More evidence is needed to support their efficacy in treating sensitivity.3
RESIN-BASED MATERIALS
As previously noted, a protective smear layer forms as a by-product of NSPT. This initially embeds at different depths into open dentinal tubules, reducing the permeability and sensitivity of the underlying dentin.4 The presence or absence of the smear layer can affect the effectiveness of resin-based dentin bonding agents.4 Some bond by penetrating and embedding into the smear layer, while others require complete removal for bonding. Restorative resins tend to require a dry, mineralized surface for optimal bonding, therefore, the composition of dentin poses an adhesive challenge. The presence of the material within the collagen matrix and dentinal tubules provides a means of mechanical retention for the resin by creating a hybrid layer including resin “tags” that interlock into the tooth structure.4 Additionally, some bonding agents are able to provide two treatment modalities at once by adding a desensitizer to the bonding agent. This treatment option includes an etchant to prepare the tooth, which may necessitate the use of local anesthesia, unless a self-etch adhesive is available.21
Resin-based agents include resin-modified glass ionomer varnish, fluoride-containing self-etch adhesive, and glutaraldehyde-containing etch-and-rinse adhesive. In-office applied resin-based materials may provide sensitivity relief for up to 6 months.22
LASER THERAPY
Laser treatment is thought to use two mechanisms to address sensitivity. First, lasers occlude dentinal tubules by melting and fusing the tubules or smear layer by altering the protein structure. Second, lasers are used to reduce nerve stimulation.4 The Nd:YAG laser is the most studied, but diode lasers and laser-emitting toothbrushes have also been evaluated.2 More evidence is needed on this subject.23
PERIODONTAL SURGERY AND ENDODONTIC THERAPY
Periodontal surgery is sometimes used to treat sensitivity.24 The amount of recession, keratinized tissue, and vestibular depth, as well as esthetic concerns surrounding the sensitive tooth should be considered before determining whether periodontal surgery is appropriate.25 Reduction in sensitivity is not frequently evaluated in studies regarding periodontal surgery outcomes. A few studies have shown significant improvement in reducing pain associated with thermal stimuli, which merit additional investigation. In general, patients prefer more cost-effective and less invasive procedures to address sensitivity.24 On average, a root-coverage procedure completely heals within 2 weeks, so depending on the extensiveness of sensitivity following NSPT, this may be an appropriate option.26
Anatomically, dental pulp is innervated by pulpal nerve supply, which is enclosed by the dentin. Changes or stimuli in the oral environment trigger multiple types of nerve stimuli. Tooth pulp cannot accommodate increases in volume because it is unable to swell. If there is an ongoing, chronic stimuli inducing an inward direction of fluid flow, then inflammatory mediators are stimulated, which can lead to the typical short, sharp pain of sensitivity as well as long-lasting, dull tooth pain. If the normally reversible sensitivity persists, allowing the pulp to become irreversibly inflamed, complete pulpal necrosis can occur.27 Root canal therapy can alleviate neuronal impulse and pain.4 However, less invasive measures should be attempted first before considering endodontic therapy.27
CLINICAL IMPLEMENTATION
During NSPT appointments, patient-specific oral hygiene instruction should be provided and informed consent must be obtained.1 Patients need to weigh the benefit of treatment with the potential risk of developing sensitivity. If sensitivity persists beyond a few weeks post-NSPT, patients should return for clinical evaluation, diagnosis, and assessment of location and severity of symptoms. Once a diagnosis is determined, at-home therapies can be recommended.4 If the sensitivity persists following the at-home regimen, or the need for a multifactorial approach is identified, then in-office professional recommendations should be explored.4 As with all clinical procedures, the final step is appropriate documentation in the health record, including signs/symptoms, initial diagnosis, treatment rendered, post-operative instructions, any additional recommendations, follow-up/reassessment plan for subsequent appointments, and appropriate referrals if applicable.
Clinicians have a variety of options for addressing tooth sensitivity caused by NSPT, which should help patients manage or eliminate any recurring signs and symptoms.
![]() REFERENCES
REFERENCES
- Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol. 1997;24:808–813.
- Draenert ME, Jakob M, Kunzelmann K-H, Hickel R. The prevalence of tooth hypersensitivity following periodontal therapy with special reference to root scaling: a systematic review of the literature. Am J Dent. 2013;26:21–27.
- West NX, Seong J, Davies M. Management of dentine hypersensitivity: efficacy of professionally and self-administered agents. J Clin Periodontol. 2015;42:S256–S302.
- Hargreaves K, Goodis H, Tay F. Seltzer and Bender’s Dental Pulp. 2nd ed. Batavia, Illinois: Quintessence Publishing Co Inc; 2012.
- Asnaashari M, Moeini M. Effectiveness of lasers in the treatment of dentin hypersensitivity. J Lasers Med Sci. 2013;4:1–7.
- Johannsen A, Emilson CG, Johannsen G, Konradsson K, Lingström P, Ramberg P. Effects of stabilized stannous fluoride dentifrice on dental calculus, dental plaque, gingivitis, halitosis and stain: a systematic review. Heliyon. 2019;5:e02850.
- Troil BV, Needleman I, Sanz M. A systematic review of the prevalence of root sensitivity following periodontal therapy. J Clin Periodontol. 2002;29:173–177.
- Lin YH, Gillam DG. The prevalence of root sensitivity following periodontal therapy: a systematic review. Int J Dent. 2012;2012:407023.
- Fischer C, Wennberg A, Fischer RG, Attstrom R. Clinical evaluation of pulp and dentine sensitivity after supragingival and subgingival scaling. Endod Dent Traumatol. 1991;7:259–265.
- Tammaro S, Wennstrom J, Bergenholtz G. Root-dentin sensitivity following non-surgical periodontal treatment. J Clin Periodontol. 2000;27:690–697.
- Gernhardt CR. How valid and applicable are current diagnostic criteria and assessment methods for dentin hypersensitivity? An overview. Clin Oral Investig. 2013;17:S31–S40.
- Garcia-Godoy F, Trushkowsky R. A diagnostic device to record dentin hypersensitivity. Am J Dent. 2013;26:3B–4B.
- Bubteina N, Garoushi S. Dentine hypersensitivity: a review. Available at: longdom.o/g/open-access/dentine-hypersensitivity-a-review-2161-1122-1000330.pdf. Accessed December 11, 2020.
- Grossman LI. A systematic method for the treatment of hypersensitive dentin. J Am Dent Assoc. 1935;22:592–602.
- Li Y, Suprono M, Mateo LR, et al. Solving the problem with stannous fluoride: Extrinsic stain. J Am Dent Assoc. 2019;150:S38–S46.
- Zhu M, Li J, Chen B, et al. The effect of calcium sodium phosphosilicate on dentin hypersensitivity: a systematic review and meta-analysis. PLoS ONE. 2015;10:1–15.
- Cummins D. Dentin hypersensitivity: from diagnosis to a breakthrough therapy for everyday sensitivity relief. J Clin Dent. 2009;20:1–9.
- Marieb E. Human Anatomy and Physiology. 7th ed. San Francisco: Pearson Benjamin Cummings; 2007.
- Poulsen S, Errboe M, Lescay Mevil Y, Glenny AM. Potassium containing toothpastes for dentine hypersensitivity. Cochrane Database Syst Rev. 2006;3:CD001476.
- Marto CM, Paula AB, Nunes T, et al. Evaluation of the efficacy of dentin hypersensitivity treatments—a systematic review and follow-up analysis. J Oral Rehab. 2019;46:952–990.
- Ide M, Morel AD, Wilson RF, Ashley FP. The rôle of a dentine-bonding agent in reducing cervical dentine sensitivity. J Clin Periodontol. 1998;25:286–290.
- Luiz de Oliveira da Rosa W, Lund RG, Piva E, Fernandes da Silva A. The effectiveness of current dentin desensitizing agents used to treat dental hypersensitivity: a systematic review. Quintessence Int. 2013;44:535–546.
- Sgolastra F, Petrucci A, Severino M, Gatto R, Monaco A. Lasers for the treatment of dentin hypersensitivity: a meta-analysis. J Dent Res. 2013;92:492–499.
- Chambrone L, Tatakis D. Periodontal soft tissue root coverage procedures: a systematic review from the AAP Regeneration Workshop. J Periodontol. 2015;86:8–51.
- Newman MG, Takei H, Klokkevold P, Carranza F. Newman and Carranza’s Clinical Periodontology. 11th ed. Amsterdam: Elsevier; 2012.
- Burkhardt R, Hämmerle CHF, Lang NP. Self-reported pain perception of patients after mucosal graft harvesting in the palatal area. J Clin Periodontol. 2015;42:281–287.
- Longridge NN, Youngson CC. Dental pain: dentine sensitivity, hypersensitivity and cracked tooth syndrome. Prim Dent J. 2019;8:44–51.
From Dimensions of Dental Hygiene. January 2021;19(1):16-18,21.