 GOPIXA/ISTOCK/GETTY IMAGES PLUS
GOPIXA/ISTOCK/GETTY IMAGES PLUS
Saliva’s Role in Remineralization
Various preventive therapies are available to encourage remineralization and inhibit demineralization.
The development of dental caries is a multifactorial, dynamic process. The constant cycle of the demineralization/remineralization processes can be positively or negatively impacted by a variety of oral and systemic factors. Assessing caries risk status and identifying appropriate remineralization therapies for those deemed high risk are key to improving patients’ oral health.
An important consideration in caries risk assessment and remineralization therapy selection is saliva. It is a critical biological and protective factor in the remineralization of enamel.1 Saliva’s buffering capacity and flow of secretion are directly related to the rate and extent of demineralization.2 Saliva can neutralize acids, form a protective membrane on tooth surfaces, and enhance remineralization by providing calcium, phosphate, and fluoride to enamel and dentin.3 The pH level of saliva directly affects remineralization through the amount of calcium and phosphate ions available to the enamel via saliva in times of acidic challenge.4Saliva can act as a replenishing source and inhibit tooth demineralization during periods of low pH, while promoting tooth remineralization when the pH returns to a neutral state.5 Systemic conditions, hereditary disorders, a variety of medications, and other medical interventions can negatively affect salivary production, buffering potential, and the amount of calcium and phosphate available for remineralization. As such, salivary flow should be a regular component of individual caries risk assessment at preventive visits.
DEMINERALIZATION PROCESS
Demineralization is the process of removing mineral ions from hydroxyapatite crystals of hard tissues, such as enamel, and when unchecked, can result in caries development.5–7 Demineralization is a continuous, cyclical process that includes remineralization, during which saliva plays a key role.5,7 The demineralization process takes place on the tooth surface when biofilm—consisting of both bacterial plaque and the pellicle—is present on the enamel and dentin.8 When fermentable carbohydrates are ingested, Lactobacillus and Streptococcus bacteria in the biofilm metabolize the carbohydrates and produce acid.7 This acid is then able to diffuse across the tooth surfaces, dissolving the minerals in the enamel and dentin.7
Although demineralization results in the loss of mineral ions, it can be reversed during remineralization. Both processes occur on the tooth surface, however, a considerable number of mineral ions must be lost from hydroxyapatite before cavitation occurs.5 The extent of demineralization and remineralization depends on several factors, including both the amount of calcium and phosphate available and the pH levels of the saliva. Those with reduced salivary flow tend to have more acidic saliva and biofilm, raising the risk for continued demineralization and eventual cavitation.4,9
Patients with reduced salivary flow and/or compromised buffering potential need assistance with the remineralization piece of the cycle. While it is important to prevent early lesions, identifying caries lesions in the early stages of the demineralization process while the condition is still reversible is critical. Oral health professionals can intervene with preventive remineralization therapies before the process becomes irreversible. The International Caries Detection and Assessment System (ICDAS) is a way to visually assess the development of early caries lesions on coronal, smooth, and root surfaces (see the web version of this article for tables on the ICDAS system for lesion and root lesion identification).8,10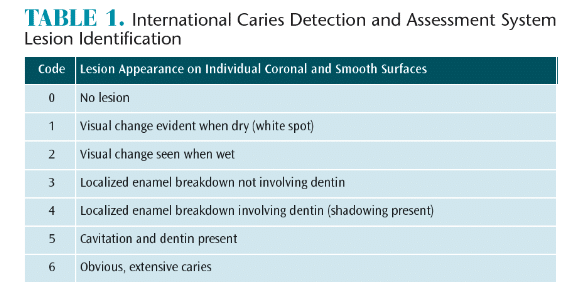
HYPOSALIVATION
Hyposalivation and/or xerostomia is a decrease in salivary flow and is characterized by oral dryness.11 It can be caused by medications; autoimmune diseases, such as Sjögren’s syndrome; radiotherapy/chemotherapy; and hormone disorders.11Approximately 30% of patients between the ages of 20 and 69 have xerostomia.12 Medications are the most common cause of dry mouth.13 As life expectancy grows, the prevalence of dry mouth increases, due to the number of medications prescribed for various health conditions.13 A review by Smith and Burtner14 revealed dry mouth was the most common side effect (80.5%) of 200 of the most frequently prescribed medications in the United States. Patients who experience diminished salivary production are at an increased risk for oral diseases such as caries and/or mucosal infections.11
The protective qualities of saliva depend on volume. These protective qualities can be significantly enhanced or decreased, depending on the rate of secretion in unstimulated and stimulated conditions.1 Unstimulated, normal salivary secretion is > 0.3 ml/minute with ranges from 0.5 L per day to 1.5 L per day, compared with ranges of 0.1 ml/minute to 0.7 ml/minute in patients with reduced salivary production.3 Decreased salivary flow creates an oral environment that inadequately neutralizes acids, increasing the intraoral pH for long periods.11 Any decrease in salivary volume should be monitored, as it can significantly shift the balance in caries risk. Additionally, other risk factors—such as frequent consumption of acidic drinks and eating a high-sugar diet—can accelerate demineralization in an already compromised environment.
ASSESSING SALIVARY FLOW RATE
Assessment of salivary gland function should be included in routine dental visits, as it is essential in diagnosing salivary gland hypofunction as the cause of xerostomia.3,15,16 Clinicians generally use a subjective approach to identify and assess dry mouth, such as a patient’s response to a health questionnaire or medical history form.3 However, an objective measurement of qualitative or quantitative changes in saliva is ideal and best captured by collecting whole saliva or saliva from individual glands.17
Saliva is produced and secreted from the major and minor salivary glands. The major salivary glands are the parotid, submandibular, and sublingual. Individual major salivary gland collection can be captured with a modified Carlson-Crittenden device for the parotid gland, and a modified Wolff saliva collector for the submandibular and sublingual glands.3 Whole saliva can be collected in either an unstimulated or stimulated method. Both are collected with a tube and a funnel.3 Methods used to stimulate whole saliva flow include gum base, paraffin wax, rubber bands, and citric acid.3 The ability to assess salivary flow rate chairside enables clinicians to identify patients experiencing salivary gland hypofunction and develop personalized treatment options to support the remineralization process.
ANALYZING SALIVA SAMPLES
Once saliva samples have been collected, chairside and laboratory tests are available for further analysis. These tools can be used to evaluate saliva pH, as well as saliva buffering capacity.18 Research conducted by Singh et al18 evaluated the use of the saliva-check buffer kit, systronics electrode pH meter, saliva-check mutans kit, and semiautoanalyser in identifying caries risk in children. Results indicated that the mean values for pH level, buffering capacity, and calcium and phosphorous ions were higher in children without caries, compared with children with active caries lesions.18 Anand et al19 measured pH value and buffering capacity of saliva using a handheld pH meter after hydrochloric acid titration. The results revealed a significant relationship between pH level and dental caries experience. These studies show chairside and/or laboratory saliva testing can be included in routine dental examinations as a noninvasive method to help predict caries risk.18,19
TREATMENT OPTIONS
Several saliva substitutes and stimulants are available for patients with salivary gland hypofunction in the form of sprays, gels, oils, mouthrinses, and chewing gums.11 Each option differs with respect to the base substance, chemical composition, viscosity, and patient preference.11 Other treatment options directly impact the remineralization process. There is conflicting research as to whether one therapy is better than another.20 Oral health professionals need to be knowledgeable about the differences in these options, so a customized treatment plan can be created to lower caries risk and increase remineralization.
Certain salivary substitutes have reportedly increased demineralization by significantly decreasing intraoral pH.21–23 Aykut-Yetkiner et al11 found that four saliva substitutes increased demineralization due to low pH or the presence of citric acid found in the substitute. However, several saliva substitutes were found to significantly increase remineralization due to the high-viscous consistency of the products, leading to a mechanical protection of the tooth surface.11 Patients with xerostomia may want to use high-viscous saliva substitutes and avoid saliva substitutes with low pH or citric acid.11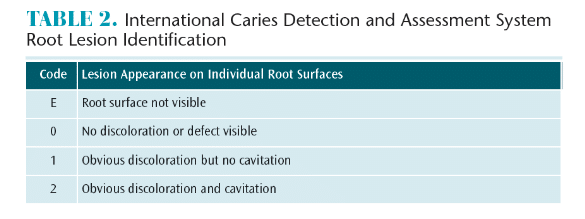
The use of professionally applied fluoride in combination with at-home fluoride products may enhance remineralization. Poor salivary contact and limited accessibility to interproximal surfaces make it difficult to manage incipient caries lesions.24 Songsiripradubboon et al24 investigated the remineralization properties of fluoride mouthrinses used at different times in conjunction with fluoride toothpaste on incipient caries lesions. Results showed that twice daily use of 0.05% sodium fluoride mouthrinse combined with twice daily use of fluoride toothpaste yielded the greatest remineralization of incipient caries.24
The remineralization process in patients with reduced salivary production is often hindered and the use of fluoride can be limited by the lack of calcium and phosphate ions present.25 Fluoride, calcium, and phosphate are needed to aid in the remineralization process during a cariogenic attack.26 Amorphous calcium phosphate (ACP), a combination of soluble salts of calcium and phosphorous, may help to remineralize tooth structure.27 Peric et al28 evaluated the effect of casein phosphopeptide-ACP (CPP-ACP) and casein phosphopeptide-amorphous calcium fluoride phosphate (CPP-ACFP) pastes in patients with Sjögren’s syndrome. Results indicated that patients who used these pastes experienced a slight increase in salivary pH values, a significant rise in plaque pH values, and partial or complete occlusion of enamel defects. Evidence suggests that pastes containing CPP-ACP/CPP-ACFP enhance remineralization in patients with Sjögren’s syndrome.26 Mendes et al29 evaluated the effect of paste containing CPP-ACP and paste containing CPP-ACP combined with fluoride in the remineralization of white-spot lesions. Results found the highest rates of remineralization occurred with the use of CPP-ACP combined fluoride paste. This clinical trial suggests CPP-ACP pastes containing fluoride may increase the efficacy of CPP-ACP in the remineralization process.29
Other options used to support remineralization include calcium sodium phosphosilicate (NovaMin) and tri-calcium phosphate (TCP). NovaMin is composed of calcium, sodium, phosphorus, and silica, and is designed to release calcium and phosphate, enhancing remineralization.30 TCP is used in collaboration with fluoride and may support remineralization better than fluoride alone.31
CONCLUSION
Saliva is an important biological and protective factor in the remineralization process.1 The pH level of saliva, along with the amount of available calcium and phosphate,directly impact demineralization during the times of acidic challenge.4 Early caries lesions can be detected and reversed with the help of noninvasive chairside tools to assess salivary production, pH levels, and buffering capacity. Additionally, several saliva substitutes are available for patients experiencing salivary gland hypofunction in the form of sprays, gels, oils, mouthrinses, and chewing gums to aid in remineralization.11 Oral health professionals must be knowledgeable about the various preventive therapies available to enhance remineralization, and use this information to create personalized treatment plans to lower caries risk and improve patients’ oral health status.
REFERENCES
- Hara AT, Zero DT. The potential of saliva in protecting against dental erosion. Monogr Oral Sci. 2014;25:197-205.
- Meurman JH, ten Cate J. Pathogenesis and modifying factors of dental erosion. Eur J Oral Sci. 1996;104:199–206.
- Navazesh M, Kumar SK. Measuring salivary flow: challenges and opportunities. J Am Dent Assoc. 2008;139:35S-40S.
- Aiuchi H, Kitasako Y, Fukuda Y, et al. Relationship between quantitative assessments of salivary buffering capacity and ion activity product for hydroxyapatite in relation to cariogenic potential. Aust Dent J. 2008;53:167–171.
- Abou Neel EA, Aljabo A, Strange A, et al. Demineralization-remineralization dynamics in teeth and bone. Int J Nanomedicine. 2016;11:4743–4763.
- Creeth, JE, Karwal R, Hara AT, et al. A randomized in situ clinical study of fluoride dentifrices on enamel remineralization and resistance to demineralization: effects of zinc. Caries Res. 2018;52:129–138.
- Featherstone JDB. Dental caries: a dynamic disease process. Aust Dent J. 2008;53:286–291.
- Pretty IA, Ellwood RP. The caries continuum: opportunities to detect, treat, and monitor the remineralization of early caries lesions. J Dent. 2013;41:S12–521.
- Aranibar Quiroz EM, Alstad T, Campus G, et al. Relationship between plaque pH and different caries-associated variables in a group of adolescents with varying caries prevalence. Caries Res. 2014;48:147–153.
- Shivakumar KM, Prasad S, Chandu GN. International caries detection and assessment system: a new paradigm in detection of dental caries. J Conserv Dent. 2009;12:10–16.
- Aykut-Yetkiner A, Wiegand A, Attin T. The effect of saliva substitutes on enamel erosion in vitro. J Dent. 2014;42:720–725.
- Flink H, Bergdahl M, Tegelberg A, et al. Prevalence of hyposalivation in relation to general health, body mass index and remaining teeth in different age groups of adults. Community Dent Oral Epidemiol. 2008;36:523–531.
- Delli K, Spijkervet FK, Kroese FG, et al. Xerostomia. Monogr Oral Sci. 2014;24:109–125.
- Smith RG, Burtner AP: Oral side-effects of the most frequently prescribed drugs. Spec Care Dentist. 1994;14:96–102.
- Ship JA, Fox PC, Baum BJ. How much saliva is enough? “normal” function defined. J Am Dent Assoc. 1991;122:63–69.
- Navazesh M. Methods for collecting saliva. Acad Sci. 1993;694:72–77.
- Navazesh M, Christensen CM. A comparison of whole mouth resting and stimulated salivary measurement procedures. J Dent Res. 1982;61:1158–1162.
- Singh S, Sharma A, Sood PB, et al. Saliva as a prediction tool for dental caries: an in vivo study. J Oral Biol Craniofac Res. 2015;5:59–64.
- Anand S, Masih U, Yeluri R. Comparative quantitative assessments of salivary ion activity product for hydroxyapatite and buffering capacity in children with different caries experience. J Clin Pediatr Dent. 2016;40:480–485.
- Furness S, Glenny AM, Worthington HV, et al. Interventions for the treatment of oral cavity and oropharyngeal cancer: chemotherapy. Cochrane Database Syst Rev. 201l;4:CD006386.
- da Silva Marques DN, da Mata AD, Patto JM, et al. Effects of gustatory stimulants of salivary secretion on salivary pH and flow in patients with Sjögren’s syndrome: a randomized controlled trial. J Oral Pathol Med. 2011;40:785–792.
- Jensdottir T, Buchwald C, Nauntofte B, et al. Erosive potential of calcium-modified acidic candies in irradiated dry mouth patients. Oral Health Prev Dent. 2010;8:173–178.
- Jensdottir T, Nauntofte B, Buchwald C, et al. Effects of sucking acidic candy on whole-mouth saliva composition. Caries Res. 2005;39:468–474.
- Songsiripradubboon S, Hamba H, Trairatvorakul C, et al. Sodium fluoride mouthrinse used twice daily increased incipient caries lesion remineralization in an in situ model. J Dent. 2014;42:271–278.
- Reynolds EC. Casein phosphopeptide-amorphous calcium phosphate: the scientific evidence. Adv Dent Res. 2009;21:25–29.
- Oliveira PRA, Coutinho TCL, Portela MB, et al. Influence of biofilm formation on the mechanical properties of enamel after treatment with CPP-ACP crème. Braz Oral Res. 2017;31:e84.
- Dorozhkin SV. Amorphous calcium (ortho) phosphates. Acta Biomater. 2010;6:4457–4475.
- Peric T, Markovic D, Petrovic B, et al. Efficacy of pastes containing CPP-ACP and CPP-ACFP in patients with Sjögren’s syndrome. Clin Oral Investig. 2015;19:2153–2165.
- Mendes AC, Restrepo M, Bussaneli D, et al. Use of casein amorphous calcium phosphate (CPP-ACP) on white-spot lesions: randomised clinical trial. Oral Health Prev Dent. 2018;16:27–31.
- Burwell AK, Litkowski LJ, Greenspan DC. Calcium sodium phosphosilicate (NovaMin): remineralization potential. Adv Dent Res. 2009;21:35–39.
- Karlinsey RL, Mackey AC, Walker ER, Frederick KE. Preparation, characterization, and in vitro efficacy of an acid-modified β-TCP material for dental hard-tissue remineralization. Acta Biomater. 2010;6:969–978
From Dimensions of Dental Hygiene. May 2018;16(5):26,28-29.


[…] production also helps in the process of remineralization. Saliva clears the path for more minerals to be added to the teeth. However, chewing gum is a […]
[…] Rinse with warm water, either plain or mixed with unrefined, mineral-rich sea salt. Mineral-rich saliva contributes to tooth remineralization, which is a normal biological response to ongoing demineralization from acidic foods, etc. (source 1, source 2) […]
[…] Rinse with warm water, either plain or mixed with unrefined, mineral-rich sea salt. Mineral-rich saliva contributes to tooth remineralization, which is a normal biological response to ongoing demineralization from acidic foods, etc. (source 1, source 2) […]