
Reduce the Risk
Improve your infection control protocol by implementing these recommendations for inanimate objects in the dental operatory.
The number of severe infections and deaths attributed to drug-resistant microorganisms, such as methicillin resistant Staphylococcus aureus (MRSA), is growing.1 One in 10 hospitalized patients in the United States acquires an infection by one of these microorganisms.1 However, little is known about the dental operatory as a source of acquired infection.2 Dental patients who have healthy immune systems can fight the microorganisms that cause health care-acquired infections but immuno compromised patients are at risk. And the treatment of medically complex patients in the dental office is increasing.3,4 Thus, reducing the risk of disease transmission in the dental office is important to ensuring the safety of all patients.5
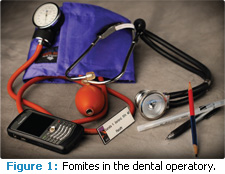
WHAT ARE FOMITES?
Fomites are inanimate objects that have the potential to transmit infectious microorganisms. Fomites in the dental operatory include pens, computer mice and keyboards, chair controls, faucet handles, cabinetry and handles, name tags, clothing, blood pressure cuffs, paper towel and soap dispensers, bib chains, instrument trays, and items on countertops (Figure 1). Fomites in nonclinical areas include magazines, toys, clipboards, pens, plants, and telephones.
Oral health care workers may not realize that some of these items can transmit microorganisms and they may inadvertently touch fomites without adequate disinfection between patients. Hands are the biggest culprit in cross contamination and cause many health care acquired infections.2,6-10
RESERVOIRS IN DISEASE TRANSMISSION
Diseases can be transmitted in four ways:
- Direct contact with microorganisms (contact with infectious tissues or fluids)
- Indirect contact (contact with contaminated objects or instruments)
- Droplet transmission (spray or spatter settling on mucous membranes)
- Airborne transmission (inhaled aerosols)5,11,12

Diseases are transmitted by microorganisms.5,12 Microorganisms need a place to live and grow (a mucous membrane or the bloodstream) and they require a way to escape the reservoir (through a cut, a mucous membrane, or an aerosol). Once the microorganism leaves its reservoir, it must find a new portal of entry (a break in the skin, a mucous membrane, a sharps injury). Reservoirs include contaminated equipment and people.9 Infections can be spread from patient to patient, operator to patient, or patient to operator. This chain of infection must happen in order to transmit disease to a susceptible host. Removing or breaking one of these links is the basis of infection control.5,12
Microorganisms can survive for days, weeks, and months on inanimate objects and surfaces where they will continue to spread.6-8 Infectious microorganisms found on fomites include influenza, hepatitis B, rotavirus, norovirus, fungi (yeast/Candida albicans), Staphylococcus (including MRSA), Enterococcus, and Clostridium difficile.2,6-10,13-27 Fomites with infectious microorganisms found in hospitals and medical clinics include pens, surgical markers, uniforms, stethoscopes, blood pressure cuffs, name tags, computer mice and keyboards, mobile communication devices, and toys.9,10,14-18,24,25,27
Stethoscopes are considered “high touch” fomites and are frequently contaminated.9,14 In fact, one study found 100% of stethoscopes (N=55) in a pediatric medical clinic were colonized with various microorganisms; 7.3% contained MRSA.16 Another study found MRSA on 32% of the stethoscopes (N=50) of emergency medical services providers.15 Various microorganisms were found on 80% of stethoscopes (N=200) from four different hospitals; four contained MRSA.17 Blood pressure cuffs can also contain MRSA. One study found 58% of blood pressure cuffs (N=24) in an English hospital were contaminated with a variety of microorganisms including MRSA and Clostridium difficile, which causes mild to life-threatening gastrointestinal infections.19 Name badges of both clinical and nonclinical staff were contaminated in a Canadian hospital. Of the 118 badges sampled, 13.6% had significant contamination with S. aureus.18 MRSA has also been found on pens and permanent markers.9,20

Computer mice and keyboards and mobile communication devices, such as cellular telephones, email devices, and personal digital assistants, can harbor pathogenic microorganisms.21-23 Medical staff in hospitals may strictly adhere to infection control standards related to jewelry, hair, and clothing in the operating room environment, but then carry around mobile communication devices.21 A study found 50% of physicians and nurses (N=100) ignored restriction policies and carried their cellular telephones during patient care activities in a hospital setting.22 This study found 10% of those health care workers’ cellular telephones were contaminated with various microorganisms, including MRSA.22 Cellular telephones carried by patients can also harbor various microorganisms. One study found 62% of cellular telephones (N=400) contained various microorganisms, including MRSA.23
THE DENTAL OPERATORY
 Oral health care workers are in close proximity to patients and the risk for disease transmission is high. Aerosols from handpieces, ultrasonic scalers, and air polishers are generated during various procedures.11,13,28,29 Aerosols and spatter were once thought to travel and settle on surfaces within 3 feet of the patient, but possible contaminated areas most likely encompass the entire operatory and beyond.11,13,28,29 Inadequate storage in the dental operatory may lead to keeping items or supplies on operatory surfaces that can easily be contaminated (Figure 2A).28 Supplies and unnecessary items should be kept in closed cabinets and off operatory surfaces to avoid potential contamination (Figure 2B). Unit dose supplies needed for procedures should be gathered ahead of time so oral health care workers will not need to reach into drawers or cabinets. If items need to be retrieved from drawers or cabinets, an overglove or transfer forceps must be used.5 Using bare hands after removing gloves is not acceptable unless hands are washed with soap and water or an alcohol hand gel.5,12
Oral health care workers are in close proximity to patients and the risk for disease transmission is high. Aerosols from handpieces, ultrasonic scalers, and air polishers are generated during various procedures.11,13,28,29 Aerosols and spatter were once thought to travel and settle on surfaces within 3 feet of the patient, but possible contaminated areas most likely encompass the entire operatory and beyond.11,13,28,29 Inadequate storage in the dental operatory may lead to keeping items or supplies on operatory surfaces that can easily be contaminated (Figure 2A).28 Supplies and unnecessary items should be kept in closed cabinets and off operatory surfaces to avoid potential contamination (Figure 2B). Unit dose supplies needed for procedures should be gathered ahead of time so oral health care workers will not need to reach into drawers or cabinets. If items need to be retrieved from drawers or cabinets, an overglove or transfer forceps must be used.5 Using bare hands after removing gloves is not acceptable unless hands are washed with soap and water or an alcohol hand gel.5,12
The role of the environment and fomites in the spread of health care-acquired infections is controversial.6,13 Research shows that contaminated fomites have caused a norovirus outbreak in a school setting, but they have not been directly implicated in disease outbreaks in health care-acquired infections or in the dental setting.5,6,27 Fomites are thought to be indirectly related to health care-acquired infections with hands serving as the main vector for transmitting infectious microorganisms.6 The hands of health care workers are the most common route of disease transmission in health care-acquired infections.2,6-10 However, patients who are colonized with MRSA carry it all over their body and clothing. Keeping the dental operatory free of extraneous items, using surface barriers, and routine disinfection may help reduce the potential for contamination by fomites and hands.
HAND HYGIENE
 Hand hygiene procedures must be performed at the beginning of the day, before leaving the treatment room, when hands are visibly soiled, after contact with potentially contaminated fomites, before and after patient care procedures, and before and after gloving since transient bacterial counts remain high.5,12 Hands must be washed with soap (plain or antimicrobial) when visibly soiled. Alcohol hand gels can be used for hand hygiene when hands are not visibly soiled. Table 1 provides a comparison of the two methods. Alcohol hand gels are growing in popularity and improving compliance because they are easy to use.5 Adequate amounts of alcohol hand gel must be used and hands must be rubbed together vigorously for at least 30 seconds.5 If hands feel dry in less than 30 seconds, too little product was used.
Hand hygiene procedures must be performed at the beginning of the day, before leaving the treatment room, when hands are visibly soiled, after contact with potentially contaminated fomites, before and after patient care procedures, and before and after gloving since transient bacterial counts remain high.5,12 Hands must be washed with soap (plain or antimicrobial) when visibly soiled. Alcohol hand gels can be used for hand hygiene when hands are not visibly soiled. Table 1 provides a comparison of the two methods. Alcohol hand gels are growing in popularity and improving compliance because they are easy to use.5 Adequate amounts of alcohol hand gel must be used and hands must be rubbed together vigorously for at least 30 seconds.5 If hands feel dry in less than 30 seconds, too little product was used.
Hand hygiene procedures leave hands dry, so using creams or lotions is recommended at the end of the day.5,12 Artificial nails should not be worn because they increase the risk of health care-acquired infections.5,12 Rings should not be worn because they are additional reservoirs for microorganisms and increase the risk of glove tears.5,12 Intact skin is a barrier to infection; cuts or abrasions act as portals of entry.5 Practicing good hand and nail care helps keep skin intact.
PERSONAL PROTECTIVE EQUIPMENT
Personal protective equipment (PPE) includes gloves, masks, eyewear, and protective clothing (eg, a lab coat). Clothing worn under protective garments, such as scrubs, is not considered PPE.5,12,26 The protective garment should be long sleeved with a high neck to protect skin and clothing from splash and spatter, should not be worn outside of patient treatment areas, and should be changed when visibly soiled.5,12 MRSA has been found on the uniforms of health care workers but not implicated in disease transmission.6,26 When possible, clothing worn under protective garments should not be worn outside of the workplace and should be laundered separately from general household laundry. Laundering of protective garments is the employer’s responsibility.5
Masks and eyewear protect mucous membranes from splash and spatter. Eyewear should be disinfected between patients. Eyewear or masks should never be touched with contaminated hands or gloves, and masks should be changed between patients or if they become moist. 5,12,26,27,29 High counts of microorganisms have been found on masks of oral health care workers using a high speed handpiece after just 40 minutes.28 Masks must be worn under face shields.5,12 Reusing masks or putting them in pockets spreads microorganisms and contaminates hands and clothing. Hair should be pulled back and protected from spatter.28
Gloves are the best protection for hands and should fit well. It is important to wash hands or use an alcohol hand gel before and after glove use because gloves have microscopic pinholes that can transmit microorganisms.5,12 High counts of transient bacteria remain on the hands and thrive in the warm, moist environment inside of a glove.12 The right gloves should be used for specific tasks, such as heavy utility gloves when handling contaminated instruments during sterilization, exam gloves during patient care, overgloves if retrieving items from drawers, and sterile surgical gloves for surgical procedures.5,12 The proper use of gloves helps reduces the potential for disease transmission to patients.5,12
MANAGING SURFACES IN THE OPERATORY
There are two types of environmental surfaces in the dental operatory: clinical contact surfaces and housekeeping surfaces. Clinical contact surfaces include items frequently touched during treatment (instruments, devices, and equipment) that require sterilization or disinfection between patients. Housekeeping surfaces are not directly touched during treatment and only require routine cleaning.5
Sterilization is a process that kills all bacterial spores under high heat and is used for critical items such as dental instruments, burs, and handpieces.5 Disinfection is a process that eliminates most microorganisms but does not kill all spores and is used for equipment or surfaces that cannot be moved or heat sterilized.5 Acceptable choices for managing operatory surfaces include the use of surface barriers, disinfection, or both.
 Dental instruments need to be sealed and stored in closed cabinets or drawers away from treatment areas because of potential aerosol or spatter contamination.5 The use of instrument cassettes can also contribute to the safety of dental instrument management. A cassette of sterilized instruments is placed chairside. After the procedure is completed, the cassette is taken to the main processing area for sterilization. The entire cassette and its contents are then cleaned in an ultrasonic cleaner or instrument washer and set to dry. After the cassette is dry, it must be wrapped to ensure that the instruments do not become contaminated after sterilization. A chemical indicator should be added to the cassette before it is wrapped so the success of the sterilization process can be determined.30
Dental instruments need to be sealed and stored in closed cabinets or drawers away from treatment areas because of potential aerosol or spatter contamination.5 The use of instrument cassettes can also contribute to the safety of dental instrument management. A cassette of sterilized instruments is placed chairside. After the procedure is completed, the cassette is taken to the main processing area for sterilization. The entire cassette and its contents are then cleaned in an ultrasonic cleaner or instrument washer and set to dry. After the cassette is dry, it must be wrapped to ensure that the instruments do not become contaminated after sterilization. A chemical indicator should be added to the cassette before it is wrapped so the success of the sterilization process can be determined.30
SURFACE DISINFECTION
Surface disinfectants are easy to use and relatively inexpensive.5 Many products are available and choosing one requires some research. There are three types of surface disinfectants: low level, intermediate level, and high level (see Table 2 for a comparison). Different types of surfaces in various settings require different levels of disinfection.
Low-level disinfectants carry a “hospital disinfectant” claim, which means the product is used on noncritical housekeeping surfaces not contaminated by blood or other bodily fluids.5,12 Low-level disinfectants are effective against the test organisms S. aureus, Salmonella, and Pseudo monas and are effective against human immunodeficiency virus (HIV) and hepatitis B.5 Hospitals generally use low-level disinfectants on most surfaces because they are not generating aerosols and spatter as in the dental setting.
Intermediate-level disinfectants also carry a “hospital disinfectant” claim but they also are effective against the hearty test organism Mycobacterium tuberculosis. Intermediate-level disinfectants are effective for use on items or surfaces contaminated with blood or other bodily fluids. 5,12 Intermediate-level disinfectants should be used on all surfaces in the dental operatory because of the aerosols and spatter of blood and other fluids. Using one product improves staff compliance and can be more cost-effective. As long as the label claims tuberculocidal activity, it is considered an intermediate-level disinfectant.
High-level disinfectants (glutaraldehyde) are not used for routine surface disinfection of operatory surfaces. They are indicated for use on semi-critical items that cannot be heat sterilized and must be submerged for 3 hours to 10 hours.5
Surface disinfectants require cleaning or removing blood or other bodily fluids first with a spray-and-wipe method.5 Next the surface must be sprayed again and left wet or damp for the time specified by the manufacturer (varies between 3 minutes and 10 minutes).

SURFACE BARRIERS
Surface barriers are alternatives or adjuncts to surface disinfection (Figure 3). Barriers are placed on clean surfaces at the beginning of the day and are changed between patients. The surface underneath does not need to be disinfected as long as the barrier is not compromised. Barriers should be changed with each patient and they are recommended for difficult-to-clean items or equipment, such as light handles, switches, chair controls, X-ray tube heads, and exposure buttons. The use of surface barriers can speed up operatory turnaround time.5,12 PPE must be worn when using disinfectants or removing contaminated barriers. Research shows the use of barriers vs disinfection alone increases infection control compliance and decreases contamination.2
BOTTOM LINE
It is impossible to eliminate all sources of infection but prudent infection control procedures and awareness can drastically reduce the risk of disease and cross-contamination. Recommendations for reducing cross contamination include: use of high velocity evacuation to reduce aerosols, use of rubber dams, use of HEPA filters in ventilation systems, use of preprocedural rinses, unit dose dispensing items for procedures ahead of time, not storing items on operatory surfaces, storing items in closed cabinets or drawers, use of handsfree equipment, disinfecting mobile communication devices with antibacterial wipes and making wipes available for patients, disinfecting blood pressure cuffs and stethoscopes, and routine disinfection of all operatory surfaces and potential fomites in clinical and nonclinical areas of the dental office. 13,23,28,29 Understanding the role of fomites in disease transmission and current infection control standards are key to breaking the chain of infection.
REFERENCES
- Biddle C. Semmelweis revisited: hand hygiene and nosocomial disease transmission in the anesthesia workstation. AANA J. 2009;77:229-237.
- Kurita H, Kurashina K, Honda T. Nosocomial transmission of methicillin-resistant Staphylococcus aureus via the surfaces of the dental operatory. Br Dent J. 2006;201:297-300.
- Mann PC. Training future dental clinicians an integrated approach to health care. NY State Dent J. 2009;75:32-35.
- Radfar L, Lakshmanan S. Medical profile of a dental school patient population. J Dent Educ. 2007;71:682-686.
- From Policies to Practice: OSAP’s Guide to the Guide lines: Workbook. Annapolis, Md: OSAP; 2004.
- Hota B. Contamination, disinfection, and cross- colonization: are hospital surfaces reservoirs for nosocomial infection? Clin Infect Dis. 2004;39: 1182-1189.
- Boyce JM. Environmental contamination makes an important contribution to hospital infection. J Hosp Infect. 2007;65(Suppl 2):50-54.
- Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6:130.
- Wolfe DF, Sinnett S, Vossler JL, Przeporia J, Engebretson BG. Bacterial colonization of respiratory therapists’ pens in the intensive care unit. Respir Care. 2009;54:500-503.
- Hartmann B, Benson M, Junger A, et al. Computer keyboard and mouse as a reservoir of pathogens in an intensive care unit. J Clin Monit Comput. 2004;18:7-12.
- Harrel SK, Molinari J. Aerosols and splatter in dentistry; A brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135:429-437.
- Molinari JA, Harte JA. Cottone’s Practical Infection Control in Dentistry. 3rd ed. Baltimore, Md: Lippincott, Williams, & Wilkins; 2010.
- Rutala WA, Weber DJ. Surface disinfection: should we do it? J Hosp Infect. 2001;48(Suppl A):S64-S68.
- Williams C, Davis DL. Methicillin-resistant Staphylococcus aureus fomite survival. Clin Lab Sci. 2009;22:34-38.
- Merlin MA, Wong DL, Pryor PW, Rynn K, Marques-Baptista A, Perritt R. Prevalence of methicillin-resistant Staphylococcus aureus on the stethoscopes of emergency medical services providers. Prehosp Emerg Care. 2009;13:71-74.
- Cohen HA, Amir J, Matalon A, Mayan R, Beni S, Barzilai A. Stethoscopes and otoscopes—a potential vector of infection? Fam Pract. 1997;14:446-449.
- Smith MA, Matthewson JJ, Ulert IA, Scerpella EG, Ericsson CD. Contaminated stethoscopes revisited. Arch Intern Med. 1996;156:82-84.
- Ota K, Profiti R, Smaill F, Matlow AG, Smieja M. Identification badges: a potential fomite? Can J Infect Control. 2007;22:162, 165-166.
- Walker N, Gupta R, Cheesbrough J. Blood pressure cuffs: friends or foe? J Hosp Infect. 2006;63:167-169.
- Tadiparthi S, Shokrollahi K, Juma A, Croall J. Using markers pens on patients:a potential source of cross infection with MRSA. Ann R Coll Surg Engl. 2007;89:661-664.
- Brady RR, Fraser SF, Dunlop MG, Paterson- Brown S, Gibb P. Bacterial contamination of mobile communication devices in the operative environment. J Hosp Infect. 2007;66:397-398.
- Goldblatt JG, Krief I, Klonsky T, et al. Use of cellular telephones and transmission of pathogens by medical staff in New York and Israel. Infect Control Hosp Epidemiol. 2007;28: 500-503.
- Akinyemi KO, Atapu AD, Adetona OO, Coker AO. The potential role of mobile phones in the spread of bacterial infections. J Infect Dev Ctries. 2009;3:628-632.
- Bures S, Fishbain JT, Uyehara C, Parker JM, Berg BW. Computer keyboards and faucet handles as reservoirs of nosocomial pathogens in the intensive care unit. Am J Infect Control. 2000;28:465-471.
- Randle J, Fleming K. The risk of infection from toys in the intensive care setting. Nurs Stand. 2006;20:50-54.
- Wilson JA, Loveday HP, Hoffman PN, Pratt RJ. Uniform: an evidence review of the microbiological significance of uniforms and uniform policy in the prevention and control of healthcare associated infections. Report to the department of health (England). J Hosp Infect. 2007; 66:301-307.
- Centers for Disease Control and Prevention. Norovirus outbreak in an elementary school—District of Columbia, February 2007. MMWR Morb Mortal Wkly Rep. 2008;56:1340-1343.
- Rautema R, Nordberg A, Wuolijoki-Saaristo K, Meurman JH. Bacterial aerosols in dental practice- a potential hospital infection problem? J Hosp Infect. 2006;64:76-81.
- Li RW, Leung KWC, Sun FCS, Samaranayake LP. Severe acute respiratory syndrome and the GDP. Part III: implications for GDP’s. Br Dent J. 2004; 197:130-134.
- Harte JA, Molinari JA. Instrument cassettes for office safety and infection control. Compend Contin Educ Dent. 2007;28:596-600.
From Dimensions of Dental Hygiene. September 2010; 8(9): 36, 39-40, 42, 44, 48.

