Providing Conscious Sedation
Nitrous Oxide delivery is an important facet of pain management during dental procedures.
Nitrous oxide (N2O) is a colorless, nonflammable, low potency anesthetic gas administered in combination with oxygen (O2) to provide minimal and moderate levels of conscious sedation in the dental office. Its chemical formula is N2O and it has been used in dentistry for more than 150 years. It is odorless, nonallergenic, and innocuous when adequate oxygen is concurrently administered. As such, N2O will not irritate the pulmonary epithelium when inhaled, making it a great option in many dental specialties.1–4
The administration of N2O-O2 is primarily indicated for patients who exhibit mild to moderate dental anxiety/fear. Minimal sedation is achieved when N2O is used in concentrations less than 50%, but it can help relieve anxiety associated with the sights, sounds, and smells of the operatory, as well as reduce the gag reflex.5 N2O-O2 can help patients with controlled conditions exacerbated by stress, such as asthma, angina, or seizures.4 Most dental hygiene procedures can be completed using concentrations of less than 50%.6 In cases that require higher concentrations of N2O, dentists may elect to exceed the 50% minimal sedation level. Patients with severe dental anxiety or who require more invasive procedures may need deeper sedation. In these situations, dentists may pair N2O with oral or intravenous sedatives.
CONTRAINDICATIONS
It is difficult to find consensus regarding absolute contraindications to N2O use. Each patient should be evaluated individually, weighing the risks and benefits to identify possible relative contraindications, which are a patient’s individual circumstance that increases his or her potential for an adverse reaction. For example, N2O is contraindicated for elective treatment or prophylaxis among pregnant patients as it crosses the placental barrier, exposing the fetus. If a pregnant patient needed an emergency procedure, however, its use may be warranted after a medical consultation with her physician.4,7,8 Other relative contraindications are chronic obstructive pulmonary disease, cystic fibrosis, recent middle ear or eye surgery where a gas bubble was used, bowel obstruction, current or recovering addiction issues, psychological impairment, use of antidepressants, use of psychotropic drugs, or severe cardiovascular conditions.3,4,6,9 When used concurrently with a minimum of 30% oxygen, N2O possess no additional risks to patients and can be used in most situations when minimal sedation is indicated.4,10
There are instances, however, where N2O delivery simply will not be effective. For example, a patient must be able to breathe through his or her nose. Patients with active upper respiratory infection, rhinitis, or abnormal nasal anatomy that impairs nasal airflow may not be candidates for N2O-O2. Patients who cannot tolerate the nasal hood or experience claustrophobia are also not good candidates for administration.
ADMINISTRATION
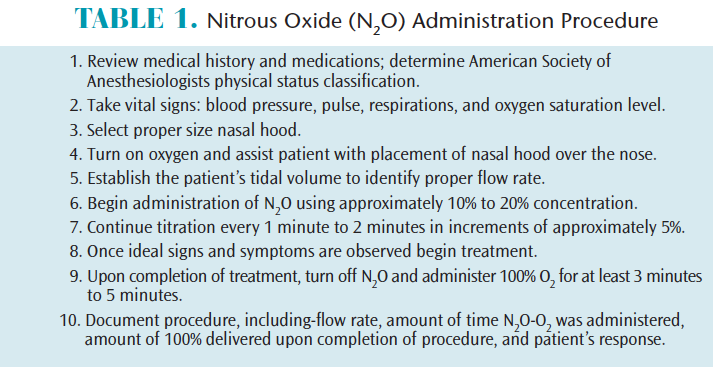
The administration of N2O–O2 is quite simple (Table 1). Always begin with a thorough review of the patient’s health history and medications to determine the patient’s American Society of Anesthesiologists (ASA) physical status classification. Obtain vital signs that include blood pressure, pulse, and respiratory rate. Additionally, the use of pulse oximetry, which includes monitoring and recording oxygen saturation of the blood at the beginning, during, and upon completion of the procedure, is prudent when delivering inhalation sedation. According to the ASA Practice Guidelines for Nonanesthesiologists, the use of the pulse oximeter is required for any patient who will have more than 50% N2O delivered, which is considered more than minimal sedation.11
N2O–O2 gas is delivered by a nasal hood that fits directly over the patient’s nose. Different sizes are available and correct size selection maximizes the benefits of the gaseous agent to the patient and minimizes leakage into the operatory. Begin the procedure by turning on the oxygen to approximately 6 L. Assist the patient, but allow him or her to place the nasal hood over his or her own nose, ensuring a comfortable and proper fit. Establish the patient’s tidal volume—which for adults is typically 6 L/min to 8 L/min and 3 L/min to 5 L/min for children—to identify the proper flow rate. If the patient is not able to breathe comfortably through the nose and feels the need to take a breath through the mouth, then increase the liters of oxygen being delivered. Begin inhalation sedation with the titration of N2O at a concentration level of 20%.4,6 Remember that sedation is a continuum, thus, it is not possible to know exactly how a patient will respond to a given concentration. Continue titration, using the “start low, go slow” mantra, by increasing N2O by 5% approximately every 1 minute to 2 minutes, making sure the established flow rate is maintained at all times. Once the proper level has been obtained and the patient is starting to show ideal signs and symptoms (Table 2) of conscious sedation, the oral health professional should maintain the desired concentration.4,12,13 Some experts suggest reducing the concentration by a few percent at this point to avoid delayed increases in intensity while establishing the baseline. Patients often refer to N2O as “laughing gas,” however, patients who display uncontrollable laughter are likely receiving too high of a concentration of N2O gas.
Once the desired level of sedation is achieved, the procedure may begin. Patients should never be left unattended during administration. Upon completion of treatment, terminate the flow of N2O, maintain the established tidal volume, and increase the O2concentration to 100% for no less than 3 minutes to 5 minutes in order to remove the increased N2O present at the alveolar sites in the lungs.4,10 If patients are still experiencing signs and symptoms, additional O2 may be necessary. This step helps avoid diffusion hypoxia, which may cause headache, nausea, or grogginess due to the reduction of oxygen reaching the body from the lungs.
Administration documentation is required in the patient’s health record. It should include vital signs, O2 saturation, established tidal volume, percent concentration of the gases, duration of N2O-O2 delivery and duration of O2 given upon completion of procedure. Documentation should also include how the patient responded to the N2O. Submit charges using the American Dental Association (ADA), current dental terminology code D9230.
HOW IT WORKS
 N2O is the least potent of the anesthetic gases, thus not all people will respond the same way. Approximately 5% of the population are considered hypo-responders, and will not feel the effects at all.3,4Another 5% of the population are hyper-responders and will feel the effects quickly.3 For inhalation anesthetics, the mechanism of action occurs through diffusion, which is dependent on a concentration gradient, whereby gas will diffuse from areas of higher concentration to areas of lower concentration. As N2O is inhaled, there is a higher concentration of N2O in the lungs, so the N2O will diffuse into the blood. This process occurs rather quickly, as N2O has one of the lowest partition coefficients of any gas mixture. N2O is referred to as relative analgesia, as it does not block or eliminate pain, but, instead, attenuates the body’s response to noxious stimuli by raising the patient’s pain threshold. Its role as an anesthetic is limited because when N2O is used as a single agent for minimal and moderate levels of conscious sedation, it is incapable of inducing general anesthesia7,9 Though there has been great progress in the understanding of N2O over the past 30 years, the analgesic, anxiolytic, and anesthetic mechanisms of action are still not fully understood.13
N2O is the least potent of the anesthetic gases, thus not all people will respond the same way. Approximately 5% of the population are considered hypo-responders, and will not feel the effects at all.3,4Another 5% of the population are hyper-responders and will feel the effects quickly.3 For inhalation anesthetics, the mechanism of action occurs through diffusion, which is dependent on a concentration gradient, whereby gas will diffuse from areas of higher concentration to areas of lower concentration. As N2O is inhaled, there is a higher concentration of N2O in the lungs, so the N2O will diffuse into the blood. This process occurs rather quickly, as N2O has one of the lowest partition coefficients of any gas mixture. N2O is referred to as relative analgesia, as it does not block or eliminate pain, but, instead, attenuates the body’s response to noxious stimuli by raising the patient’s pain threshold. Its role as an anesthetic is limited because when N2O is used as a single agent for minimal and moderate levels of conscious sedation, it is incapable of inducing general anesthesia7,9 Though there has been great progress in the understanding of N2O over the past 30 years, the analgesic, anxiolytic, and anesthetic mechanisms of action are still not fully understood.13
SAFETY
More defined regulations, equipment safety features, fail safe mechanisms, and monitoring protocols have contributed to the decrease in lethal doses and incidental occupational exposures caused by N2O.14 In 1976, the ADA Council on Dental Materials, Instruments, and Equipment adopted standards for inhalation units in the US. By 1980, the council recommended that scavenging units be installed and that monitoring protocols be in place.15 Today, operating a delivery system without a scavenging unit to decrease the amount of waste gas in the air fails to meet the minimum standard of care.10
The National Institute for Occupational Safety and Health currently recommends an exposure limit of 25 ppm in the dental operatory during administration.16 For an 8-hour time weighted average, the American Conference of Governmental Industrial Hygienists recommends a threshold limit value of 50 ppm.17 Dosimeter badges are available to monitor exposure of N2O to the provider. The accuracy and validity of these badges are questionable, as trace amounts of N2O found in the ambient air can also be recorded by these badges.
The single most important safety step is the titration method. Incremental doses of N2O delivered over a short period allow for the minimum concentration to be used while still achieving the desired result. This provides safety not only to the patient, but also decreases the waste gas exposure to the clinician.4
With any drug or agent, the potential for harm exists and risk increases with chronic, high-concentration exposures. Prolonged contact (more than 6 hours) and/or long term use of N2O has been shown to inactivate vitamin B12, causing a biochemical imbalance that may lead to bone marrow depression, megaloblastic anemia, pernicious anemia, and neurological deficits, such as peripheral neuropathies.18–21 Other studies have demonstrated that exposures, even at high levels, of 6 hours or less have not shown to cause any long-lasting physiological changes and are generally considered safe.3,4,12,22,23
CONCLUSION
Along with fluoride and local anesthesia, the administration of N2O is considered by some as one of the greatest achievements of the dental profession.24 Oral health professionals who want to add to add N2O to their armamentarium must obtain the proper education and training to deliver this safe and effective agent. State dental practice acts need to also be reviewed to ensure N2O administration is within the scope of practice.
REFERENCES
- National Center for Biotechnology Information. Compound Summary for CID 948, Nitrous Oxide. Available at: pubchem.ncbi.nlm.nih.gov/compound/948. Accessed September 27, 2017.
- Wells H. A history of the discovery of the application of nitrous oxide gas, ether and other vapors, to surgical operations. Available at: woodlibrarymuseum.org/museum/item/139/wells-h.-a-history-of-the-discovery-of-the-application-of-nitrous-oxide-gas,-ether,-and-other-vapors,-to-surgical-operations,-1847. Accessed September 27, 2017.
- Clark M, Brunick A. Handbook of Nitrous Oxide and Oxygen Sedation. 4th ed. St. Louis:Elsevier Mosby; 2015.
- Malamed S. Sedation: A Guide to Patient Management. 5th ed. St. Louis: Mosby Elsevier; 2010.
- Chidiac JJ, Chamseddine L, Bellos G. Gagging prevention using nitrous oxide or table salt: a comparative pilot study. Int J Prosthet. 2001;14:364.
- Darby M and Walsh M. Dental Hygiene Theory and Practice. 4th ed. St. Louis: Elsevier Saunders; 2015:768–785.
- Becker D, Rosenburg, M. Nitrous oxide and the inhalation anesthetics. Anesth Prog. 2008; 55:124–131.
- Crawford JS, Lewis M. Nitrous oxide in early pregnancy. Anaesthesia. 1986;41:900–905.
- Clark M. Back to the future: an update on nitrous oxide/oxygen sedation. Available at: dentalacademyofce.com/courses/2044/PDF/1103cei_nitrous.pdf. Accessed September 27, 2017.
- Shuman I. Nitrous oxide: use and safety. Available at: dentalacademyofce.com/courses/3036%2FPDF%2F1604cei_Shuman_Nitrous%20-%20updated%20web.pdf. Accessed September 27, 2017.
- American Society of Anesthesiologists Task Force on Sedation and Analgesia by Non-Anesthesiologists. Practice guidelines for sedation and analgesia by non-anesthesiologists. Anesthesiology. 2002;96:1004–1007
- Dionne R, Phero JC, Becker DE. Management of Pain and Anxiety In the Dental Office. St. Louis: WB Saunders; 2002.
- Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesthesia Progress. 2007;54:9–18.
- Whitcher CE, Zimmerman DC, Tonn, EM, Piziali, RL (1977). Control of occupational exposure to nitrous oxide in the dental operatory. J Am Dent Assoc. 1977;95:763–776.
- Nitrous oxide in the dental office. ADA Council on Scientific Affairs; ADA Council on Dental Practice. J Am Dent Assoc. 1997;128:364–365.
- National Institute for Occupational Safety and Health. Control of Nitrous Oxide in Dental Operatories. Available at: cdc.gov/niosh/docs/hazardcontrol/hc3.html. Accessed September 27, 2017.
- TLVs, Threshold Limit Values for Chemical Substances in the Work Environment. Cincinnati; American Conference of Governmental Industrial Hygienist; 1990:32.
- Lassen HCA, Henriksen E, Neukirch F, Kristensen H. Treatment of tetanus: Severe bone-marrow depression after prolonged nitrous-oxide anesthesia. Lancet. 1956;267:527–530.
- Yagiela J. Health hazards and nitrous oxide: a time for reappraisal. Anesthesia Progress. 1991;38:1-11,
- Amess J, Burman JF, Rees GM,et al. Megaloblastic haemopoiesis in patients receiving nitrous oxide. Lancet. 1978;2:339–342.
- Nunn JF, Sharer NM, Gorchein A, et al. Megaloblastic haemopoiesis after multiple short-term exposures to nitrous oxide. Lancet. 1982;1:1379–1381.
- Weimann J. Toxicity of nitrous oxide. Best Pract Res Clin Anaesthesiol. 2003; 17:47–61.
- O’Sullivan H, Jennings F, Ward K, et al. Human bone marrow biochemical function and megaloblastic hematopoiesis after nitrous oxide anesthesia. Anesthesiology. 1981;55:645–649.
- Greene NM. A consideration of factors in the discovery of anesthesia and their effects on its development. Anesthesiology.1971;35:515–522.
Featured image by ONEBLINK-CJ/ISTOCK/GETTY IMAGES PLUS
From Dimensions of Dental Hygiene. October 2017;15(10):32-34.