
Power Instrumentation Considerations
As the use of ultrasonic therapy grows, dental hygienists must be knowledgeable about how to best use this modality.
The primary objective of periodontal therapy is to remove bacterial and calculus deposits from tooth surfaces. While both ultrasonic and hand instrumentation are used in initial and periodontal maintenance therapies, power instrumentation is growing in popularity and produces similar clinical results to manual scaling.1–3 Ultrasonic instrumentation requires specific considerations for its safe and effective use, and dental hygienists need to be mindful of these factors before beginning treatment.
Periodontal debridement can be effectively accomplished with hand instruments or ultrasonic scalers, but a combination of both is most prudent.3 In order for either instrumentation technique to be effective and safe, the therapy must be applied correctly to prevent damage to the tooth surface and to provide positive clinical results. The extent of deposits, as well as the current periodontal condition, impact the type of technique needed. The first step in ultrasonic instrumentation is to identify and select an insert armamentarium based on these needs. Generally, only the active tip of the ultrasonic insert/tip (UIT), which is approximately 4 mm, functionally removes biofilm and calculus. If instrumentation is not carried out using correct angulation (0° to 15°) and adaptation (?4 mm) of the active tip, subgingival deposits may persist, and the root surface may show iatrogenic irregularities.4 The combination of excessive angulation (>15°) and heavy lateral pressure can result in significant tooth surface loss within a short period of time.5
Ultrasonic instrumentation offers significant benefits to clinicians, including: improved access to deep, narrow pockets and furcations; ergonomic advantages due to the need for only a light touch during use; and a consistent flush of water created by lavage. The wide variety of UITs is also helpful—from ultra-thin tips to inserts with LED lights. It is imperative that clinicians use the right UIT for the job at hand; using the same UIT?regardless of patients’ individual needs does not follow best practices. While the wide variety of UITs is helpful, one study showed that UITs demonstrated the most consistent outcomes when used in conjunction with ultrasonic scalers from the same manufacturer.6 However, more research is needed to support the validity of this finding.
FURCATION ACCESS
Effective instrumentation of furcation defects, mostly class II and III, is challenging due to limited accessibility to the furcation entrance and the complex morphology of molar teeth.7 Some studies indicate hand instruments alone are not adequate for removing root deposits in these areas because furcation entrances typically have smaller dimensions than the blade widths of the most commonly used curets.8,9 Furcation widths vary between maxillary/mandibular and first/second molars, as well as buccal to lingual. An average furcation entrance measures 0.76 mm, whereas the blade widths of various curets average between 0.7 mm and 1.10 mm. Alternately, UITs designed for furcation access have an approximate diameter of 0.56 mm. Adequate space is needed to allow the active tip of the UIT enough movement against the root surface. If the blade has the same width as the furcation, it will not provide enough distance to achieve an effective stroke for the removal of biofilm and calculus.9 Periodontal therapy often fails in molar furcations, thus the use of UITs designed to access furcations may provide the greatest efficacy in initial periodontal therapy.7
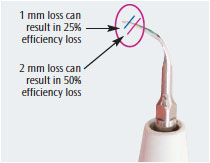
INFLUENCE OF TIP WEAR
Tip wear is a significant consideration in the efficacy of any power-driven instrument. During clinical use, UITs wear down, resulting in loss of length at the tip. The loss of 1 mm in tip length translates to a 25% loss of efficiency due to decreases in oscillation and amplitude. A reduction of 2 mm in length cuts efficiency by half. At this point, the UIT should be replaced.10,11 Figure 1 illustrates the effects of tip wear. Recently, Arabaci et al11 reported that tip wear significantly influenced root surface roughness, especially when used at higher tip angulation and power settings. Wear at the tip of any instrument results in the operator changing the angle, using more force or strokes, and, in the case of ultrasonic instruments, increasing the power setting.10 Avoiding the use of worn UITs is especially important when treating patients in periodontal maintenance because they are treated three times to four times per year. Therefore, UITs should be periodically evaluated for wear and replaced when indicated.10
IMPLANT CARE
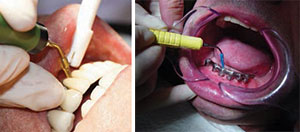
Dental implant therapy is popular in dentistry today, and instruments used for deposit removal must be compatible with the implant surface. Effective self-care and regular maintenance visits are essential to prevent peri-implant mucositis and peri-implantitis (inflammatory reactions in the tissues surrounding an implant).12 Traditional hand instruments and UITs may cause implant surface and titanium abutment irregularities that, in turn, contribute to biofilm and calcified deposit accumulation.13 Plastic scaling instruments are available for use around implants and abutments, but recent literature suggests these instruments (both hand and power) may produce irregularities in the implant surfaces and are ineffective in biofilm and calculus removal.14 UITs are now available with single-use soft tips that fit over the metal ends (Figure 2A and Figure 2B). They are designed to effectively remove biofilm and light calculus without damaging the surface of the implant or the peri-implant mucosa.
AEROSOL PRODUCTION
While ultrasonic instrumentation is a popular therapy in the delivery of nonsurgical periodontal care, it is not without risks. Studies have confirmed that aerosols contaminated with bacterial, viral, and fungal pathogens are produced during ultrasonic and sonic scaling.14 Though the level of harm from aerosolized microbes during ultrasonic scaling has not been established, minimizing clinician and patient exposure must be a top priority to ensure protection from these potentially infectious agents.15 The use of a preprocedural mouthrinse can significantly reduce the numbers of microorganisms in the oral cavity. Over-the-counter antiseptic mouthrinses, such as those containing essential oils or cetylpyridinium chloride, are effective, as are prescription broad-spectrum antimicrobial rinses, such as 0.12% chlorhexidine. These products reduce viable microbes in aerosols when used immediately prior to the start of dental procedures for an adequate length of time (30 seconds or more).16,17
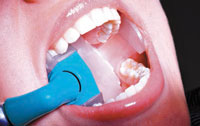
Another precautionary consideration is the use of a high-volume evacuator (HVE) during ultrasonic scaling to protect against aerosol contamination. The manual implementation of a HVE to help control aerosols may be difficult without the help of an assistant; therefore choosing a short or half-length HVE tip may facilitate retraction and manipulation during ultrasonic debridement.18 The use of a hands-free device (Figure 3) is an additional option for HVE delivery.
INTERACTION BETWEEN ULTRASONIC DEVICES AND CARDIAC PACEMAKERS
Ultrasonic scaling instruments can cause electrical interference with cardiovascular implantable electronic devices, such as pacemakers, due to the electromagnetic energy they emit. The use of ultrasonic scaling on patients with pacemakers is controversial, and there is conflicting evidence about whether and to what extent ultrasonic instruments may impede these devices. Researchers agree that piezoelectric scalers do not exert negative effects on cardiovascular implantable electronic devices.19,20
Modern pacemakers have built-in features to protect them from electrical interference that may be encountered during daily routines.21 According to the American Heart Association, dental equipment poses little or no risk for interference with a pacemaker. However, research is needed to further assess this risk.22 Nonetheless, patients with an implanted cardiac pacemaker need to stay at least 50 cm away from a magnetostrictive device to ensure their safety.22 Patients with an implantable device should always carry their pacemaker identification cards. Oral health professionals should document the following in the patient’s record: manufacturer of the device, model number, serial number, date of implantation, and mode of operation. Each pacemaker manufacturer provides detailed instructions about what sources of electromagnetic sources should be avoided, and dental hygienists can use this information to identify and record precautions recommended for a particular cardiac device.23
SUMMARY
Mechanical disruption of biofilm remains the foundation of nonsurgical periodontal therapy. Ultrasonic scaling offers the ability to thoroughly instrument deep pockets and furcation areas, as well as provide highly effective and efficient care to those undergoing initial or maintenance periodontal therapy. But, as with any modality, its efficacious use requires careful technique and education. Dental hygienists are charged with remaining up to date on best practices for power instrumentation.
REFERENCES
- Chapper A, Catão VV, Oppermann RV. Hand and ultrasonic instrumentation in the treatment of chronic periodontitis after supragingival plaque control. Braz Oral Res. 2005;19:41–46.
- Christgau M, Männer T, Beuer S, Hiller KA, Schmalz G. Periodontal healing after non-surgical therapy with a new ultrasonic device: a randomized controlled clinical trial. J Clin Periodontol. 2007;34:137–147.
- Ioannou I, Dimitriadis N, Papadimitriou K, Sakellari D, Vouros I, Konstantinidis A. Hand instrumentation versus ultrasonic debridement in the treatment of chronic periodontitis: a randomized clinical and microbiological trial. J Clin Periodontol. 2009;36:132–141.
- Marda P, Prakash S, Devaraj CG, Vastardis S. A comparison of root surface instrumentation using manual, ultrasonic and rotary instruments: an in vitro study using scanning electron microscopy. Indian J Dent Res. 2012;23:164–170.
- Lea SC, Felver B, Landini G, Walmsley AD. Ultrasonic scaler oscillations and tooth-surface defects. J Dent Res. 2009;88:229–234.
- Lea SC, Walmsley AD. Do ultrasonic scaler inserts and generators from the same manufacturer optimize performance? Annual Clinical Journal of Dental Health. 2011;1:22–27.
- Claffey N, Polyzois I. Non-surgical therapy. In: Lindhe J, Lang NP, Karring T, eds. Clinical Periodontology and Implant Dentistry. 5th ed. Oxford, United Kingdom: Wiley-Blackwell; 2008:770–772.
- Marcaccini AM, Pavanelo A, Nogueira AV, Souza JA, Porciuncula HF, Cirelli JA. Morphometric study of the root anatomy in furcation area of mandibular first molars. J Appl Oral Sci. 2012;20:76–81.
- dos Santos KM, Pinto SC, Pochapski MT, Wambier DS, Pilatti GL, Santos FA. Molar furcation entrance and its relation to the width of curette blades used in periodontal mechanical therapy. Int J Dent Hyg. 2009;7:263–269.
- Lea SC, Landini G, Walmsley AD. The effect of wear on ultrasonic scaler tip displacement amplitude. J Clin Periodontol. 2006;33:37–41.
- Arabaci T, Cicek Y, Dilsiz A, Erdogan IY, Kose O, Kizidaq A. Influence of tip wear of piezoelectric ultrasonic scalers on root surface roughness at different working parameters. A profilometric and atomic force microscopy study. Int J Dent Hyg. 2013;11:69–74.
- Curylofo F, Barbosa LM, Roselino AL, Fais LM, Vaz LG. Instrumentation of dental implants: a literature review. Revista Sul-Brasileira de Odontologia. 2013;10(1):82-88.
- Fakhravar B, Khocht A, Jefferies SR, Suzuki JB. Probing and scaling instrumentation on implant surfaces: an in vitro study. Implant Dent. 2012;21:311–316.
- Trenter SC, Walmsley AD. Ultrasonic dental scaler: associated hazards. J Clin Periodontol. 2003;30:95–101.
- Cristina ML, Spagnolo AM, Sartini M, et al. Evaluation of the risk of infection through exposure to aerosols and splatters in dentistry. Am J Infect Control. 2008;36:304–307.
- Gupta DG, Mitra DD, KP DA, et al. Comparison of efficacy of pre-procedural mouth rinsing in reducing aerosol contamination produced by ultrasonic scaler: a pilot study. J Periodontol. 2013 Jul 15. Epub ahead of print.
- Brockman L, Smith D. Control the aerosol risk. Dimensions of Dental Hygiene. 2013;11(10):32–38.
- Devker NR, Mohitey J, Vibhute A, et al. A study to evaluate and compare the efficacy of preprocedural mouthrinsing and high volume evacuator attachment alone and in combination in reducing the amount of viable aerosols produced during ultrasonic scaling procedure. J Contemp Dent Pract. 2012;13:681–689.
- Roedig JJ, Shah J, Elayi CS, Miller CS. Interference of cardiac pacemaker and implantable cardioverter-defibrillator activity during electronic dental device use. J Am Dent Assoc. 2010;141:521–526.
- Brand HS, Entjes ML, Nieuw-Amerongen AV, van der Hoeff EV, Schrama TA. Interference of electrical dental equipment with implantable cardioverter-defibrillators. Br Dent J. 2007;203:577–579.
- Cohan L, Kusumoto FM, Goldschlager NF. Environmental effects on cardiac pacing systems. In: Kusumoto FM, Goldschlager NF, eds. Cardiac Pacing for the Clinician. 2nd ed. New York: Springer Publishing Co; 2008:595.
- American Heart Association. Devices that may interfere with pacemakers. Available at: heart.org/HEARTORG/Conditions/Arrhythmia/Prevention TreatmentofArrhythmia/Devices-that-may-Interfere-with-Pacemakers_UCM_302013_Article.jsp. Accessed March 24, 2014.
- Yeo TP, Berg NC. Counseling patients with implanted cardiac devices. Nurse Pract. 2004;29:58,61–65.?
From Dimensions of Dental Hygiene. April 2014;12(4):20,22,24.

