 THEASIS/E+/GETTY IMAGES PLUS
THEASIS/E+/GETTY IMAGES PLUS
The Oral and Systemic Health Benefits of Arginine
Research demonstrates the effects of arginine in destabilizing biofilm, decreasing enamel demineralization and dentinal hypersensitivity, and supporting remineralization.
This course was published in the November 2019 issue and expires November 2022. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Describe the significance of arginine and its associated mechanism of action.
- Discuss arginine’s potential to promote remineralization and decrease dentinal hypersensitivity.
- Provide oral health professionals with recommendations for using arginine in clinical practice.
Dental caries, periodontal diseases, tooth erosion, and dentinal hypersensitivity are common oral health problems experienced by populations across the globe.1 Fluoride is considered the gold standard in the prevention of caries. Systemic and topical fluoride use has contributed to the declining prevalence of tooth decay.1–3 However, caries is still a widespread concern and more effective solutions should be evaluated to further reduce its prevalence.2
Arginine is a semi-essential amino acid found in the human body, composed of proteins and peptides.1 It is also readily available from dietary sources.4,5 In the past decade, evidence has suggested that arginine may be helpful in reducing biofilm accumulation, caries-like lesions, and dentinal hypersensitivity.1 For patients at high-caries risk, arginine is a promising ingredient especially when used in conjunction with fluoride.
MECHANISM OF ACTION
Dental biofilm is an organized accumulation of bacterial species within a matrix that consists of organic and inorganic materials from saliva, bacterial products, and gingival crevicular fluid.6 Biofilm communities play a major role in increasing the risk for dental caries and periodontal diseases by creating acidic by-products that demineralize tooth enamel and break down alveolar bone.7 The extent to which biofilm impacts the risk of dental caries and periodontal diseases depends on its location, biomass, microbiome composition, and spatial arrangement.6 Obstacles in treating dental biofilm communities, which contribute to the incidence of dental caries and periodontal disease, continue to be major clinical and public health challenges in the United States. Methods to control the damaging effects of biofilm on the oral cavity continue to be the subject of investigations. Fluoride is an effective tool, but it has minimal effect on disrupting oral biofilms. As biofilm is an important risk factor for both caries and periodontal diseases, the use of fluoride may be highly appropriate for a low caries-risk patient, but may not be effective for a high-risk individual with a plaque index and frequent carbohydrate intake.1 The use of arginine appears to be a promising method of improving oral conditions to decrease enamel demineralization and alveolar bone loss. It also has the potential to decrease dentinal hypersensitivity and increase remineralization in conjunction with other therapies.
The development and stability of biofilm communities is dependent on interbacterial communication systems. Arginine interrupts these cell-to-cell interactions in dental biofilm and, in doing so, reduces the biomass of pathogenic bacteria. Arginine has not been shown to have bacteriostatic or bactericidal properties that affect specific bacteria within the biofilm, but it does appear to destabilize the biofilm, depending on the extent of arginine concentration.6 In 2015, Kolderman et al6 conducted an experiment that simulated the natural oral environment by creating a flow-based microfluidic system in order to test the effect of arginine on dental biofilm. Results showed that arginine was statistically significant to destabilize biofilm based on reduction in biovolume and changes in the relative proportions of bacterial species (p < 0.05). Table 1 describes the biofilm destabilizing effects over time in concentrated flowing saliva containing arginine.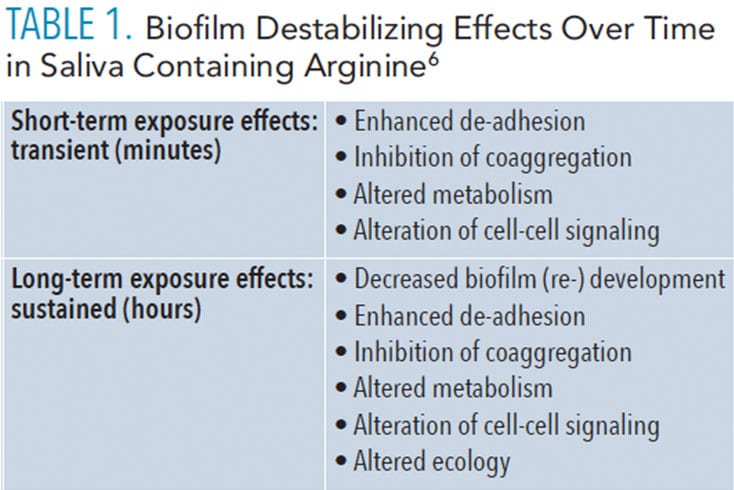
The existence of arginine in the oral cavity may also alter the bacterial architecture by affecting the production and composition of extracellular components of oral bacteria. In 2014, Sharma et al7 found that Streptococcus mutans bacterial biofilms grown without arginine had a denser extracellular matrix than biofilms grown in the presence of arginine. These extracellular membrane-bound polysaccharides of oral bacteria, such as S. mutans, play a major role in biofilm accumulation, as they affect the organization and cohesion onto both other bacteria and tooth surfaces.
Data also suggest that the addition of arginine to the oral environment may alter the biofilm species composition. Results from an in vitro experiment conducted by Kolderman et al6 also showed that supplementing saliva with 500 mm L-arginine hydrogen chloride modified the species that static biofilm community contained. The initial saliva consisted of a large proportion of Neisseria sanguis. followed by Granulicatella and Streptococcus spp. Following the addition of arginine, the saliva composition was dominated by Streptococcus spp. followed by Veillonella, Neisseria spp., and Fusobacterium spp. Neisseria bacterial species are associated with periodontal inflammation and tissue destruction, so the decrease in this species could be beneficial to the oral environment.
Arginine is metabolized via the arginine deiminase system (ADS).1 The active enzyme in the ADS system is called arginine deiminase, which is a surface enzyme found on oral bacteria, such as S. sanguinis.1 Arginine deaminase catalyzes the hydrolysis of L-arginine to L-citrulline, ornithine, carbon dioxide, adenosine triphosphate, and ammonia. The ammonia production from this catabolism reaction is beneficial in multiple ways. The accumulation of ammonia assists in neutralization of the acidic environment because it locally increases the pH. This pH increase helps to neutralize the effects of acidification from sugar metabolism. The action of this enzyme may help maintain a more neutral pH that bolsters the tenacity of bacteria with arginine deaminase, while being competitive against opportunistic bacteria that lead to the development of caries.8 Because of ammonia’s effect on pH in the oral cavity, arginine and its subsequent metabolism may increase the capacity of dental plaque biofilm to produce ammonia. This may positively influence the ability of the host to prevent or stop the progression of tooth decay.1
One limitation of arginine is that it is not bactericidal (ie, arginine has not exhibited antimicrobial properties in previous studies).6 The potential benefits of arginine in the oral cavity may be considered ecological rather than antimicrobial; it may have more impact on the environment around the bacteria than on the bacteria itself.9 This could be beneficial because the altered surrounding environment may lead to an increase in effectiveness of other products such as antimicrobials or fluoride.9 Arginine may be able to enhance the ability of antimicrobials to penetrate oral biofilms and increase the efficacy of fluoride on remineralization.6 Table 2 describes the various differences in benefits of fluoride vs arginine.1,4,6,8,10–13
REMINERALIZATION AND DENTINAL HYPERSENSITIVITY
Dental caries is a complex disease process requiring interaction between the host and bacterial, environmental, and dietary factors. Disrupting one of these risk factors may interrupt the caries process if preventive measures occur during early stages (eg, when a caries lesion has impacted only the enamel). Remineralization may be limited, however, when plaque biofilm and its resultant acidic byproducts are exposed to teeth while fluoride exposure is present. As such, conventional sodium fluoride dentifrices and rinses pose obstacles to high-risk individuals, such as children with increased biofilm indices and high sugar consumption on a regular basis.1 Primary teeth tend to be affected by dental caries more rapidly than permanent teeth due to their thinner enamel composition. Therefore, dentifrices with fast-acting, resolving components are essential for effective caries prevention, particularly in children. An advantage of arginine is its ability to form an alkaline environment in the oral cavity, creating a less favorable atmosphere for cariogenic bacteria to proliferate.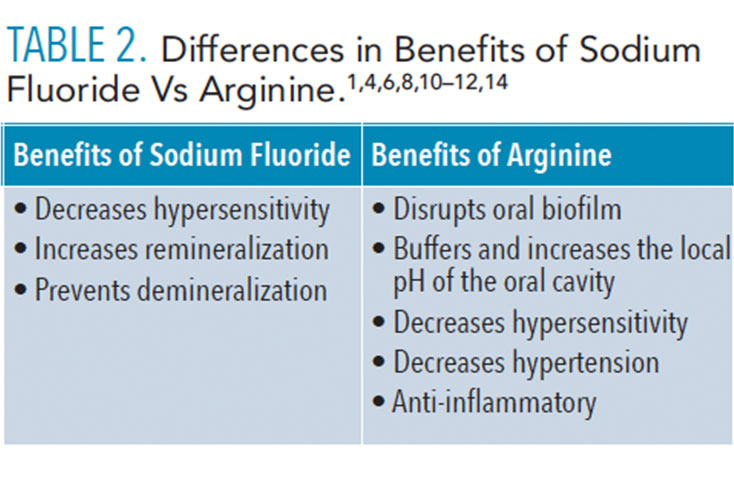
Kraivaphan et al10 explored the anti-caries efficacy of arginine dentifrices and a sodium fluoride dentifrice in children with low to moderate caries risk. The two test dentifrices contained arginine, sodium fluoride, and insoluble calcium compounds, while the control was a dentifrice with sodium fluoride alone. Prior to this clinical trial, no significant differences in the number of decayed, filled, and missing teeth (dmft) existed among the three study groups. There were no significant differences observed after 1 year of clinical trial. After 2 years, the groups using arginine-containing dentifrices showed statistically significant lower dmft increments compared to the group using control fluoride dentifrice. Results of this clinical trial suggest the potential for arginine to combat caries lesion progression in conjunction with fluoride and insoluble calcium compounds. However, this experimental study was an industry-sponsored trial, which introduces a potential for bias.
Arginine-containing fluoride dentifrices act as a natural remineralizing agent on permanent teeth. Bijle et al11 concluded that 2% arginine added to fluoride dentifrice had statistically significant remineralization effectiveness, while 4% and 8% of arginine-containing fluoride toothpaste had no statistical significance in remineralization effectiveness. Ineffectiveness of remineralization in 4% and 8% arginine-containing fluoride dentifrices could be due to reduced concentration of fluoride in the dentifrice solution. Incorporation of arginine in the fluoride dentifrice was based on a weight/weight determination, where higher concentrations of arginine correlated with a reduction in the amount of fluoride added to achieve the proper product blend. As a naturally occurring element, arginine serves as an organic method in reversing demineralization, but further research needs to be done to determine its maximum efficacy.
Research shows that arginine produces comparable desensitizing effects similar to the natural process of the saliva when combined with sodium fluoride and calcium carbonate.12 Dentinal hypersensitivity is prevalent in 8% to 74% of adults, and occurs when exposed dentin interacts with various stimuli, such as thermal or tactile irritants, which results in sharp pain until the stimuli is removed.12 The wide range of dentinal hypersensitivity prevalence could be due to contributing factors including but not limited to genetics, nutritional habits, and systemic conditions. One accepted explanation for hypersensitivity is the hydrodynamic theory, which states that certain stimuli may promote fluid motion inside of dentinal tubules, creating sharp pain responses in the nerve fibers.12 Although a variety of interventions have been used to manage dentinal hypersensitivity, it still remains a significant problem. Arginine-containing dentifrices demonstrated capability of dentinal tubule occlusion and resistance to acidic challenges and pulpal pressure, according to an in vitro study.14 In 2013, Yan et al12 explored the effects of arginine-containing dentifrice compared to placebo, potassium salt-containing, and strontium-containing dentifrices. Results indicated that arginine-containing dentifrices had better tactile and air blast assessment results than the placebo and potassium salt-containing dentifrices, but inconsistent results compared to strontium-containing dentifrice.
EXPANDING ARGININE USE IN ORAL HEALTH CARE
Naturally occurring arginine or arginine supplementation in the form of multivitamins and probiotics may support systemic and oral health. Arginine is beneficial in enhancing the circulatory, gastrointestinal, immune, and reproductive systems.13 Decreased arginine production may negatively impact these systems, leading to the development or progression of diseases.4,8
On average, American adults consume about 5 g of arginine daily and only 40% of that arginine is readily available to the body after being metabolized and/or excreted.24,13,15 According to McNeal et al,13 consuming 30 g of arginine daily can help reduce systolic blood pressure and serum glucose concentration. Arginine is used as a substrate for nitric oxide production, which plays a major role in vasodilation of blood vessels and increased vascular permeability.5,15 Patients with hypertension due to the widening of lymphatic vessels and interstitial spaces and increased inflammatory reactions are at increased risk for lymphogenic inflammatory spread with greater tissue involvement.4 Therefore, patients with hypertension may benefit from arginine supplementation along with the use of other antihypertensive medications.
Due to metabolic changes in patients with diabetes mellitus, salivary glucose levels may rise and consequently reduce the pH in the oral cavity.16,17 Decreased pH levels promote growth of cariogenic microorganisms, which may lead to demineralization and caries formation.18 Ammonia, a byproduct of arginine metabolism, helps increase the pH of supragingival biofilm and favors the growth of ADS-positive bacteria, thereby competing with the cariogenic action of microorganisms. Arginine prebiotic supplements have the potential to disrupt supragingival biofilm matrix assembly while increasing salivary pH, reducing the growth of cariogenic bacteria.8
Composite restorations commonly accumulate cariogenic bacteria, which can promote secondary caries and lead to failed restorations.19 Geraldeli et al20 developed a 7% arginine etch and rinse adhesive system with a controlled release of arginine (0.2 µmol/cm2) aimed at reducing secondary caries. This mechanism of controlled release with 7% arginine seals and protects the border between the tooth and adhesive coating for a longer period of time. Antibacterial agents, such as zinc, silver, and quaternary ammonium, have been introduced into composite resins, glass ionomer composites, and resin modified glass ionomer composites. However, these antibacterial agents contributed to augmentation of the original properties of these materials. Some antibacterial agents decreased adhesive bond strength, which has potential to increase microleakages and secondary caries at the margins of the dental restorations. On the other hand, the addition of arginine to the adhesive did not compromise the physical or mechanical properties and was able to withstand the internal loading stresses. Arginine’s antibacterial effect is able to defend against bioproducts from the metabolism of oral bacteria by increasing the local pH. Overall, the 7% arginine adhesive system helped in reducing tooth demineralization. This is the first exploratory study currently available about the arginine-based dental adhesive system.20 The ability to incorporate arginine into adhesive systems and possibly other dental materials opens new opportunities to prevent secondary caries. However, further research is needed to confirm the reproducibility of results.
CONCLUSIONS
Arginine has shown promising results in destabilizing biofilm, decreasing enamel demineralization and dentinal hypersensitivity, and supporting remineralization. In addition to arginine’s effects in the oral cavity, it has demonstrated beneficial effects systemically. Arginine’s potential to improve the delivery of oral care is promising, but further research needs to be done to validate its effectiveness and positive benefits for improved oral health outcomes. Further long-term randomized, controlled trials are needed to establish safety, efficacy, and ideal dosages and delivery mechanisms. These investigations could lead to more reliable and generalizable results that would allow oral health care professionals to make more confident, evidence-based decisions, regarding arginine’s effectiveness in professional practice.
Acknowledgements: The authors would like to acknowledge Preethy Tom, RDH, BSDH, and Leigh A. Wyatt, BSDH, MA, MS, for their assistance with this manuscript.
REFERENCES
- Li J, Huang Z, Mei L, et al. Anti-caries effect of arginine-containing formulations in vivo: a systematic review and meta-analysis. Caries Res. 2015;49:606–617.
- Cummins D. Dental caries: a disease which remains a public health concern in the 21st century—the exploration of a breakthrough technology for caries prevention. J Clin Dent. 2013;24(Spec No A):A1–A14.
- Whelton H. Overview of the impact of changing global patterns of dental caries experience on caries clinical trials. J Dent Res. 2004;83(Spec No C):C29–C34.
- Patel JJ, Miller KR, Rosenthal C, et al. When is it appropriate to use arginine in critical illness? Nutr Clin Pract. 2016;31:438–444.
- S Vasdev, V Gill. The antihypertensive effect of arginine. Int J Angiol. 200817:7–22.
- Kolderman E, Bettampadi D, Dowd SE, et al. L-Arginine destabilizes oral multi-species biofilm communities developed in human saliva. PLoS ONE. 2015;10:e0121835.
- Sharma S, Lavender S, Woo J, et al. Nanoscale characterization of effect of L-Arginine on streptococcus mutans biofilm adhesion by atomic force microscopy. Microbiology. 2014;160:1466–1473.
- Nascimento MM. Potential uses of arginine in dentistry. Adv Dent Res. 2018 ;29:98–103.
- Xue Y, Lu Q, Tian Y, et al. Effect of toothpaste containing arginine on dental plaque—a randomized controlled in situ study. J Dent. 2017;67:88–93.
- Kraivaphan P, Amornchat C, Triratana T, et al. Two-year caries clinical study of the efficacy of novel dentifrices containing 1.5% arginine, an insoluble calcium compound and 1,450 ppm fluoride. Caries Res. 2013;47:582–590.
- Bijle M, Manikandan E, Edward L, et al. The combined enamel remineralization potential of arginine and fluoride toothpaste. J Dent. 2018;76:75–82.
- Yan B, Yi J, Li Y, Chen Y, Zongdao S. Arginine-containing toothpastes for dentin hypersensitivity: systematic review and meta-analysis. Quintessence Int. 2013;44:709–723.
- McNeal CJ, Meininger CJ, Wilborn CD, et al. Safety of dietary supplementation with arginine in adult humans. Amino Acids. 2018;50:1215–1229.
- Lavender S, Petrou I, Heu R, et al. Mode of action studies on a new desensitizing dentifrice containing 8.0% arginine, a high cleaning calcium carbonate system and 1450 ppm fluoride. Am J Dent. 2010;23(Spec No A):14A–19A.
- Alexander JW, Supp DM. Role of arginine and omega-3 fatty acids in wound healing and infection. Adv Wound Care (New Rochelle). 2014;3:682–690.
- Puttaswamy KA, Puttabudhi JH, Raju S. Correlation between salivary glucose and blood glucose and the implications of salivary factors on the oral health status in type 2 diabetes mellitus patients. J Int Soc Prev Community Dent. 2017;7:28–33.
- Latti BR, Kalburge JV, Birajdar SB, et al. Evaluation of relationship between dental caries, diabetes mellitus and oral microbiota in diabetics. J Oral Maxillofac Pathol. 2018;22:282.
- Struzycka I. The oral microbiome in dental caries. Pol J Microbiol. 2014;63:127–135.
- Sarrett DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dent Mater. 2005;21:9–20.
- Geraldeli S, Soares EF, Alvarez AJ, et al. A new arginine-based dental adhesive system: formulation, mechanical and anti-caries properties. J Dent. 2017;63:72–80.
From Dimensions of Dental Hygiene. November 2019;17(10):40—43.



