 ENVIROMANTIC/E+/GETTY IMAGES PLUS
ENVIROMANTIC/E+/GETTY IMAGES PLUS
Minimize Your Risk of COVID-19
Adhering to the following recommendations can help limit the likelihood of contracting the novel coronavirus in the dental setting.
In late 2019, a novel coronavirus (SARS-CoV-2), which manifests as COVID-19 disease, emerged and rapidly changed the world. The resulting pandemic has created heightened awareness of the risks associated with oral healthcare. Dentists and dental hygienists fall into the “very high exposure risk” category, according to the Occupational Safety and Health Administration (OSHA), as they routinely perform aerosol-generating procedures.1 As such, safety recommendations have been issued by leading government and professional organizations.
Symptoms of the potentially fatal disease range from mild to extreme, and the route of transmission has been described as airborne, droplet, and droplet nuclei, which includes talking, sneezing, and coughing. Close contact, defined as less than 6 feet, facilitates transmission. It is also possible that surfaces can be contaminated, and pathogenic aerosols can remain suspended for prolonged periods.1,2 These are all concerns.
In March 2020, guidance from several stakeholder organizations recommended dental offices temporarily close for elective care and provide only emergency services in order to flatten the curve of the pandemic.3 The United States Centers for Disease Control and Prevention (CDC) issued interim guidelines to supplement its Guidelines for Infection Control in Dental Health-Care Settings–—2003, which remain the baseline guidance for infection prevention.2,4 In support of these efforts, OSHA1 and the American Dental Association (ADA)3 have also issued guidance documents, and state health departments are providing state-specific guidance. Under the authority of each states’ governor, state-specific guidance was issued on dental office closures, policies, and reopening of dental offices. New guidance from several agencies continues to emerge, making it challenging for clinicians to stay up to date.
In terms of infection prevention, prior to the COVID-19 pandemic dental teams were primarily concerned with bloodborne pathogens, rather than respiratory pathogens, such as SARS-CoV-2. Although guidance regarding respiratory pathogens (airborne transmission-based precautions) has been released since the 2003 CDC guidelines,4 these were rarely used in routine dentistry. In light of COVID-19, dental personnel are now reviewing CDC respiratory guidance and rapidly emerging interim infection prevention guidance.
Bear in mind that OSHA is a regulatory body that can investigate and impose fines for lack of compliance with standards, such as the OSHA Bloodborne Pathogens standard CFR 1910.10305 or OSHA Hazard Communication Standard CFR 1910.1200.6 By comparison, the CDC is a guidance body that provides evidence-based recommendations, but has no regulatory authority. Some state boards of dentistry adopt CDC guidance. Professional associations, such as the ADA, American Dental Hygienists’ Association (ADHA), and the Organization for Safety, Asepsis and Prevention, publish practice and professional standards, codes of ethics, and recommendations. This article focuses on the latest guidance for dental settings.2,7–9 The latest guidance from the CDC and ADHA was published on August 28, and replaces prior guidance published May 7,2 May 28,8 July 17,2 and August 4.2 Since this remains an evolving issue, it is critical to continually monitor state and federal recommendations.
Many dental procedures produce splash, spatter, and aerosols. Current CDC guidance recommends avoiding aerosol-generating procedures whenever possible.2 The ADHA recommends consulting with local officials on disease trends, and if these indicate increased incidence of infection, clinicians should consider providing emergency care only.8 The ADHA provides a “Readiness to Work Checklist” to help clinicians decide about returning to work.8 In addition, the ADA has provided interim guidance on returning to work for nonemergency care.7 The following summarizes several major components of the latest CDC, ADA, and ADHA return-to-work guidance.
COMMUNICATION AND PATIENT MANAGEMENT
The ADA recommends sending a letter outlining what patients can expect and emphasizing the office’s commitment to safety (a template of this letter can be found in the guidance document).7 The ADHA recommends communicating with the dental team regarding PPE inventories, screening, aerosol reduction, social distancing, and scheduling to allow for adequate turnaround time.8
The CDC, ADA, and ADHA all recommend telephone prescreening asking about travel, symptoms of COVID-19, and assessment of the patient’s dental condition. This allows teams to relay information about possible triage and new procedures, such as use of face coverings, minimizing people accompanying them, social distancing, and additional screening.2,7,8
At patient check-in, the CDC, ADA, and ADHA recommend that patients don face coverings (removed only during oral procedures), and again screen patients for possible COVID-19 symptoms and travel history. Teams are also advised to take a patient’s temperature with a touchless thermometer (fever is measured at ≥ 100.0˚F), and have hand sanitizer and respiratory etiquette supplies (such as tissues, wastebaskets, and signage) available. Additional recommendations include providing pens for patients to keep (or ensure they are wiped between uses), as well as wipes or other agents to disinfect high-touch areas (such as clipboards and countertops).2,7,8 Offices may consider having patients wait in their cars and call or text when they arrive, and limit movement around the facility.2,7,8 The CDC, ADHA, and ADA also advise following up with patients, or having them report any signs or symptoms of COVID-19 within 2 days after their visit.2,7,8
EQUIPMENT AND FACILITY
After an extended period of nonuse, several maintenance protocols are needed for dental equipment, such as shocking dental unit waterlines, lab testing of waterline samples to ensure safe standards, testing autoclaves with biological indicators to ensure proper functioning and sterility, checking air compressors and suction lines, and verifying the function of technology-related items, such as software updates for electronic dental record programs.2,8
Regarding reception areas, the CDC, ADA, and ADHA recommend similar procedures, including posting signs and providing supplies for respiratory and cough etiquette, limiting seating (at least 6 feet apart), installing physical barriers (eg, plexiglass dividers at the front desk), removing toys and magazines, minimizing overlapping appointments, and regularly cleaning and disinfecting high traffic areas and high-touch surfaces with an Environmental Protection Agency (EPA)-approved disinfectant.2,7,8
ADMINISTRATIVE CONTROLS
Some of the most important recommendations from stakeholder groups discuss clinical protocols while at chairside, also known as work practice controls. These include avoiding aerosol-generating procedures (particles of respirable size, <10 µm) whenever possible.2,8 In addition to appropriate PPE, clinicians are advised to use hand instrumentation, high-volume evacuation, schedule additional time for patient care, and allow adequate time for room turnaround to let aerosols settle. Additional recommendations are to forego “double booking” (ie, see one patient at a time), avoid shaking hands, using barriers to cover keyboards and similar items, limiting unnecessary supplies, equipment and fomites in treatment areas, sterilizing nitrous oxide tubing (or use disposable tubing), separating patient chairs by at least 6 feet, providing physical barriers between patients, and limiting patient volume.2,7,8
Although it is best practice at this time to avoid the use of aerosol-generating procedures, sometimes it is necessary. If it is absolutely necessary to use handpieces or ultrasonic scalers, the CDC and ADHA both recommend high-volume evacuation, use of four-handed dentistry, and/or dental dams, as well as scheduling these procedures for the end of the day.2,8 With so much information, it can be confusing to know which procedures are considered aerosol generating. At this time, the CDC cannot create a comprehensive list of aerosol-generating procedures due to lack of consensus among experts and absence of sufficient supporting data.2 It will take time for the science to catch up. In the meantime, clinicians can use their best judgement and refer to their specific state’s rules. Most experts agree that aerosol-generating procedures include the use of high speed handpieces, ultrasonic scalers, air-water syringes, and air polishing.
An additional measure is the use of preprocedural rinsing with chlorhexidine, essential oils, povidone iodine, or cetylpyridium chloride to reduce viral loads.2,8 Although there is no evidence to support this, rinsing might reduce some oral microorganisms during the provision of care. The CDC considers this an “unresolved issue.”4
ENGINEERING CONTROLS
Engineering controls are devices or equipment that help keep workers safe. In response to COVID-19 concerns, the CDC outlines several measures offices can take, such as maintaining ventilation systems for proper air flow, consulting with a heating, ventilation and air conditioning expert to investigate improving filtration, running restroom exhaust fans continually throughout the workday, and using high efficiency particulate air filters (HEPA).
Once a patient leaves, the previously recommended 15-minute wait period to allow droplets to fall before cleaning and disinfecting the room has now been lifted by the CDC,2 but confusion still exists around this topic. The ADA asserts that it is important to wait before disinfecting treatment rooms after aerosol-generating procedures.10 The association suggests there is no strong evidence to support a “one size fits all” approach, and recommends considering some form of wait period to allow aerosols to settle, depending on the type and length of the aerosol-generating procedure, use of HEPA filtration, and volume of patients.
HAND HYGIENE and UNIVERSAL SOURCE CONTROL
Hand hygiene is crucial to safe care. Poor hand hygiene can adversely affect patients, as demonstrated by the occurrence of healthcare-acquired infections in hospitals.11 Best practices include washing with soap and water for at least 20 seconds, or the use of alcohol-based hand rubs (sanitizers) with an alcohol content of at least 60%. Hand hygiene must be performed prior to donning and after removing PPE, after any contact with material or surfaces that are potentially contaminated, and before and after patient contact.2,4
In an effort to reduce risk of transmission, universal source control is recommended. Team members should wear a mask at all times while in the dental office, preferably surgical masks (as opposed to cloth) in an effort to reduce exposure to splash and spatter. Although cloth coverings are not considered adequate PPE for procedures involving patient care, they can be used for nonclinical tasks, such as clerical duties or functions not involving direct care. Hand hygiene must be performed if the clinician touches or adjusts the mask after donning. Masks should be changed if they become contaminated or wet.
As of August 28, the CDC now also recommends the use of universal eye protection without gaps (goggles or a full face shield that covers the front and sides of the face) in addition to a mask especially in areas of moderate to substantial community transmission.2,8
PERSONAL PROTECTIVE EQUIPMENT
Employers are required to provide adequate PPE in accordance with the OSHA Bloodborne Pathogens Standard,2,4,5 PPE Standard,12 and Respiratory Protection Standard.13 As noted at the outset, dentists and dental hygienists are in the “very high risk” category of contracting COVID-19,1 so the ADHA recommends using the highest levels of PPE possible.8 Any reusable PPE, such as goggles or face shields, must be cleaned between uses. Soap and water vs disinfectants are preferred, as chemicals should not be used around tissue and skin. Dental teams must receive training on when to use PPE, what type to use for the task, correct donning and doffing protocols, proper disposal or disinfection, and the limitations of PPE.2
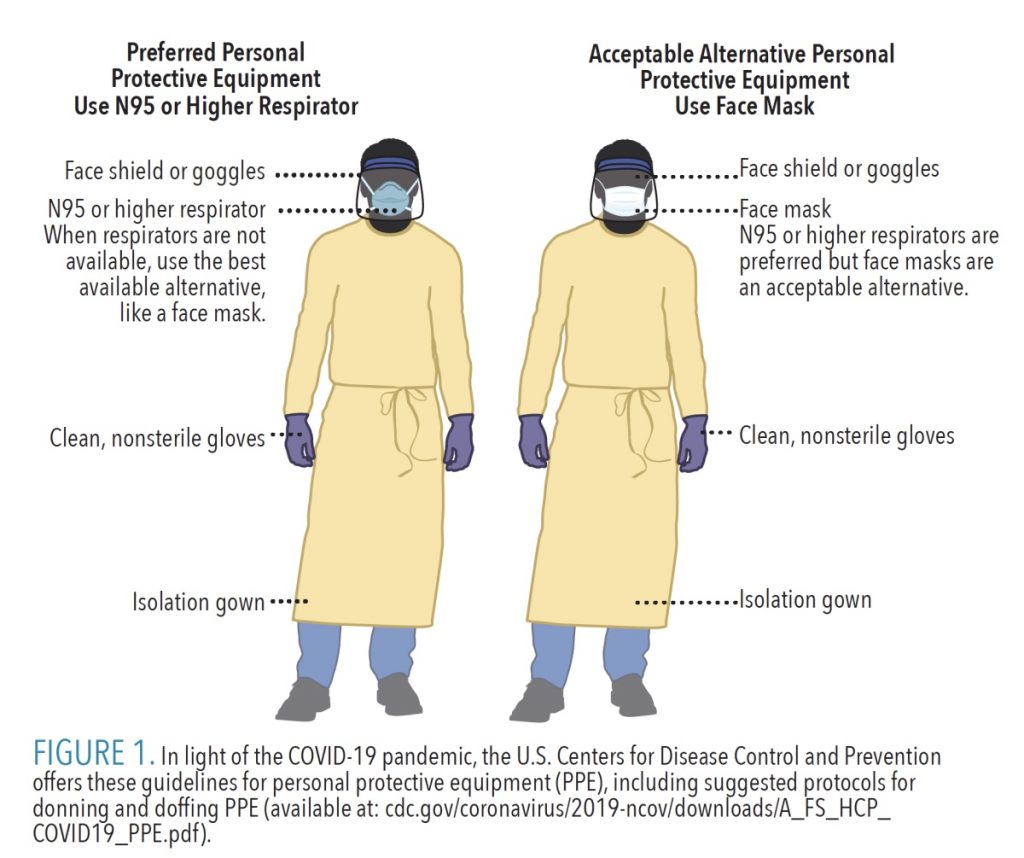
The proper PPE for any procedure involving splash or spatter includes a surgical mask (or N95 mask), eye protection (with solid side shields or a full face shield), gloves, and a gown or protective outer clothing (Figure 1).2,8 Proper PPE to use during any aerosol-generating procedure include all of the previous items, including an N95 respirator mask or other type of respirator.2,8 Prior to using an N95 mask, medical evaluation and fit testing must be conducted.2,7,8 If a surgical mask and full face shield are not available, the CDC and ADHA recommend avoiding aerosol-generating procedures.2,8
![]() OPTIMIZING SUPPLIES
OPTIMIZING SUPPLIES
Obtaining proper PPE remains a challenge. In the interim, the CDC suggests assessing current inventories of PPE, how much PPE is used/needed, working with local agencies to identify supplies, implementing engineering controls and policies, and training and educating staff on PPE use.2 In terms of extended use (or reuse) of face masks, OSHA, CDC, and ADHA provide specific guidance,8,9,13 noting this should only be employed when in “crisis capacity.” This means if there is not adequate PPE for nonurgent or elective care, those appointments should be deferred to a later date.2,8 Facilities can return to standard procedures once the PPE supply has improved.
ENVIRONMENTAL INFECTION CONTROL AND TRAINING
Guidance for operatory turnaround from the CDC and ADHA2,8 recommends additional time for proper room disinfection.2 Appropriate PPE must be worn, including heavy-duty utility gloves, eye protection, and gowns.4,8 Cleaning and disinfection recommendations outlined in the 2003 CDC guidelines should be followed.4 This involves cleaning the surfaces of bioburden to remove debris (visible or invisible), followed by the application of an intermediate-level (ie, tuberculocidal) disinfectant on the EPA List N for the appropriate contact time (per manufacturer instructions) using either a “spray-wipe-spray” or a “wipe-discard-wipe” technique.2,4,14 Routine cleaning and intermediate-level disinfectants are appropriate for protection against SARS-CoV-2. The EPA is responsible for regulating disinfectants and provides a list of products that are deemed effective.14 The ADHA suggests clinicians alternate between two rooms to allow a proper wait time before disinfection, as well as increasing the appointment length to at least 1.5 hours if only one room is available.8
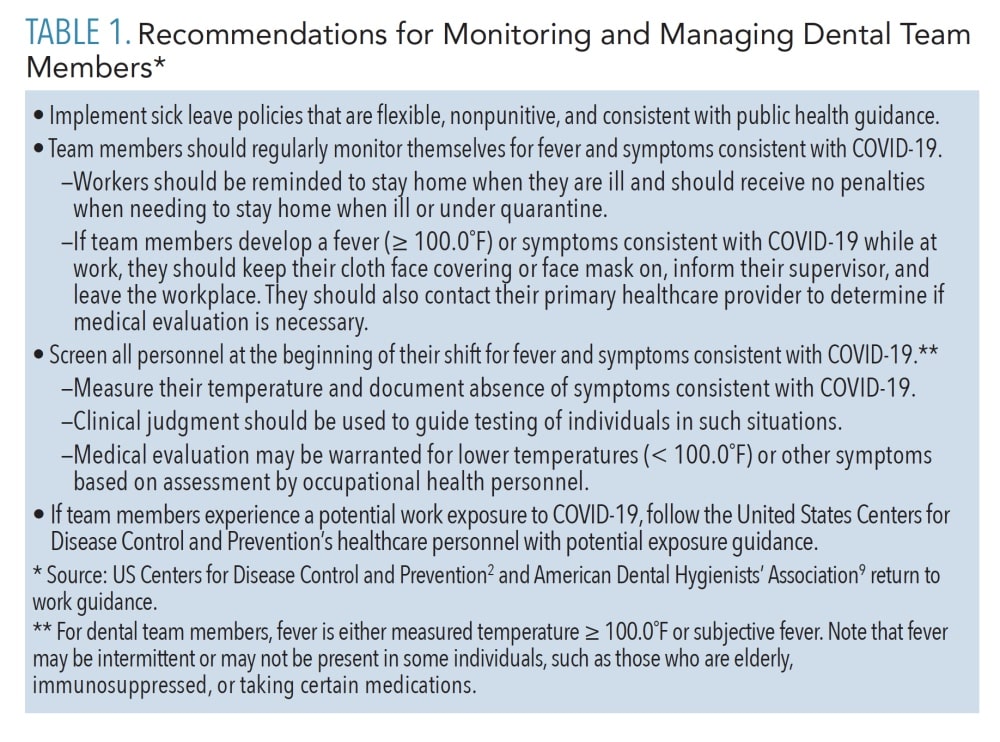
Protocols for sterilization of instruments have not changed, and the CDC continues to recommend following its 2003 guidelines, including heat sterilization of all critical and semi-critical instruments and use of biological indicators to test the functioning of autoclaves.2,4 The only change relates to nitrous oxide, as the ADA recommends using disposable nasal hoods and tubing (if possible).7 If tubing is reusable, it should be sterilized according to manufacturer instructions.8 Dental clinics should implement policies for monitoring and managing team members (Table 1).2,8 Workers must be provided with training and education related to PPE and job- or task-specific procedures where transmission might occur, refresher training on OSHA’s Bloodborne Pathogens and Hazard Communication standards, and training in any new protocols and procedures.2
CONCLUSION
In light of the COVID-19 pandemic, safety is on the minds of both providers and patients. There is a great deal of information and misinformation. Relying on credible sources is the best way to ensure safe care. Toward this goal, dental teams can rely on guidance documents and resources available from the CDC, OSHA, ADA, ADHA, and other government and professional groups.
References
- Occupational Health and Safety Administration. Guidance on Preparing Workplaces for COVD-19. OSHA 3990-03 2020. Available at: click here. Accessed September 15, 2020.
- United States Centers for Disease Control and Prevention. Guidance for Dental Settings. Interim Infection Prevention and Control Guidance for Dental Settings During the COVID-19 Response. Available at: click here. Accessed September 15, 2020.
- American Dental Association. Postponement Recommendations. Available at: click here. Accessed September 15, 2020.
- Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep. 2003;52:1–66.
- Occupational Safety and Health Administration. Bloodborne Pathogens Standard CFR 1910.1030. Available at: click here. Accessed September 15, 2020.
- Occupational Safety and Health Administration. Hazard Communication Standard CFR 1910.1200. Available at: click here. Accessed September 15, 2020.
- American Dental Association. Return to Work Interim Guidance Toolkit. Available at: click here. Accessed September 15, 2020.
- American Dental Hygienists’ Association. ADHA Interim Guidance on Returning to Work. Available at: click here. Accessed September 15, 2020.
- American Dental Association. Summary of ADA Guidance During the COVID-19 Crisis. Available at: click here. Accessed September 15, 2020.
- American Dental Association. ADA Responds to Change from CDC on Waiting Period Length. Available at: click here. Accessed September 15, 2020.
- Musu M, Lai A, Mereu NM, et al. Assessing hand hygiene compliance among healthcare workers in six intensive care units. J Prev Med Hyg. 2017;58:E231–E237.
- Occupational Safety and Health Administration. General Requirements: Personal Protective Equipment CFR 1910.132. Available at: click here. Accessed September 15, 2020.
- United States Environmental Protection Agency. List N: Disinfectants for Use Sgainst SARS-Co-V-2 (COVID 19). Available at: click here. Accessed September 15, 2020.
From Dimensions of Dental Hygiene. October 2020;18(9):22-25.