 MIKOLETTE/ISTOCK/GETTY IMAGES PLUS
MIKOLETTE/ISTOCK/GETTY IMAGES PLUS
Hypersensitivity Management With Fluoride
Fluoride varnish and silver diamine fluoride are both viable treatment options in efforts to relieve the pain of dentinal hypersensitivity, particularly among older adults.
This course was published in the June 2019 issue and expires June 2022. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Identify causes of dentinal hypersensitivity among older adults and its effects on quality of life.
- Discuss therapies for the treatment of dentinal hypersensitivity, including mechanisms of action.
- Discuss the use of sodium fluoride varnish and silver diamine fluoride (SDF) for the management of dentinal hypersensitivity.
Between 2010 and 2050, the United States population is projected to increase by 42% and by the year 2030, nearly one in five Americans will be 65 or older.1 As older adults are able to retain more teeth due to better prevention and intervention, the prevalence of dentinal hypersensitivity (DH) is expected to increase due to the high rate of gingival recession among older populations.2 DH is characterized by a short, sharp pain that arises from exposed dentin in response to thermal, tactile, or chemical stimuli.3 This pain ranges from slight to severe, and may significantly impact patients’ quality of life. The current prevalence of DH ranges from 3% to 98% depending on the diagnostic method used and the population studied.4 In older patients with periodontitis, the prevalence of DH can reach as high as 60% to 98%.2
ETIOLOGY
Gingival recession—as a result of clinical attachment loss—is the most widespread risk factor contributing to DH. Gingival recession and periodontitis are common among older adults.5 However, dentin can also become exposed through enamel loss, which may be caused by erosion, abrasion, attrition, or abfraction.3,5 When the exposed dentin comes into contact with thermal, tactile, or chemical stimuli, painful symptoms may occur.3,5 DH can also be caused by untreated caries lesions on the root surfaces. As a result of gingival recession, the root surfaces are exposed to plaque accumulation and this eventually leads to root surface caries lesions. The prevalence of root surface caries—a major oral health problem in later life—ranges from 22% to 77%.6,7
MECHANISMS OF ACTION
Many theories have been used to describe the mechanism of DH. The direct innervation theory suggests that the nerve’s endings enter the dentin through the pulp and extend to the dentinoenamel junction.8,9 Then, mechanical stimuli directly transmit the pain. As nerves have not been reliably identified in the superficial dentin, this theory does not have widespread support.
The odontoblast receptor theory suggests that odontoblasts act as receptors of pain and conduct signals to the pulpal nerves.8,9 However, this theory is not widely accepted because it is now known that the odontoblasts are not capable of producing nerve impulses.9 No synapses have been found between odontoblasts and pulpal nerves.
The most widely accepted theory behind DH is the hydrodynamic theory, which describes the mechanism of DH as shifts in fluid in response to stimuli across exposed dentinal tubules that mechanically activate the nerves located in the outer layers of the pulp.9 This movement of fluid stimulates baroreceptors and leads to neural discharge, resulting in painful sensations.9
ORAL HEALTH-RELATED QUALITY OF LIFE
Oral conditions, such as DH, can lead to physical, psychological, and social disability. Oral health-related quality of life describes the way individuals’ oral health affects their self-esteem and ability to eat, sleep, and engage in social interactions.10,11 Research shows that symptoms of DH can negatively influence a patient’s oral health-related quality of life.2,11,12 A study by Bekes et al2 found that the most common limitations included eating, drinking, and oral hygiene habits, and that cold stimuli caused the most discomfort. Patients may report discomfort when consuming cold food or beverages, report changes in dietary habits, avoid or alter oral hygiene practices, or require local anesthetic for professional prophylaxis appointments. Patients’ oral health-related quality of life should be considered when deciding when and how to treat their DH symptoms.
DIAGNOSIS AND MANAGEMENT
Diagnosis of DH involves reviewing the patient’s medical history and performing a thorough clinical examination to determine the cause and severity of symptoms. Reviewing the medical history involves asking questions about when the patient’s symptoms started, the intensity and stability of pain, and the factors that increase or decrease symptoms.8 Some techniques used to identify DH include the use of air and water, which mimic the stimulation factors and allow the clinician to determine the severity of symptoms.8 Palpation can be used to diagnose pulpitis or periodontal involvement, and transillumination and/or bite tests can be used to diagnose a fractured tooth, ruling out any other causes of pain.8
DH management ranges from noninvasive, self-performed therapy to in-office treatments using professional materials and techniques.5 Treatment focuses on reducing fluid flow in the dentin tubules, blocking the dentin tubules, and preventing the nerve response in the pulp.3,13 DH treatment should start with noninvasive, reversible, nonhazardous, and inexpensive options and progress to more invasive interventions if symptoms do not improve.5 In-office procedures may include topical application of desensitizing agents, mucogingival root coverage procedures, laser therapy, restorative, and/or pulpal therapy.5
A survey of dental professionals revealed that the most common recommendations for first-line treatments of DH are sensitivity toothpastes and desensitizing agents.14 More invasive treatments, such as restorative care or gingival grafts, were recommended as first-line of treatment by 6.6% of surveyed providers. The most popular second-line treatment recommendations were restorative care, desensitizers, or sensitivity toothpastes.14
FLUORIDE VARNISH
Sodium fluoride varnishes were approved by the US Food and Drug Administration (FDA) in the 1990s for the treatment of DH.15 Research shows that 5% sodium fluoride (NaF) varnish can be effective in treating DH.16,17 When NaF varnish is applied to hypersensitive dentin surfaces, the formation of calcium fluoride crystals block the open dentinal tubules, controlling the permeability of the exposed dentin.17–19 This, in turn, reduces the painful symptoms associated with DH. Although NaF varnishes have been approved for the treatment of DH, they are typically used off-label for caries prevention.
Because NaF varnish adheres to the tooth’s surface for hours, it is able to penetrate deep into the dentinal tubules and obstruct tubule fluid movements, which relieves symptoms of DH.13 Research shows that NaF varnish provides an immediate relief of DH symptoms after a single application.4 However, it does not have a long-term success because the layer formed by the varnish is easily removed by routine oral hygiene practices.4 NaF varnishes may be appropriate for treating DH caused by exposed dentinal tubules due to gingival recession, abrasion, or erosion.13
The application of NaF varnish takes about 4 minutes, depending on the number of teeth present.20 Because the varnish sets when it contacts saliva, it is not necessary to thoroughly dry the teeth.20 Wiping the teeth off with gauze or a cotton roll is sufficient. Once dried, the clinician applies approximately 0.3 mL to 0.5 mL of varnish directly onto the tooth’s surface with a disposable mircobrush.20 The patient is instructed to follow the manufacturer’s post-treatment recommendations regarding eating, drinking, and oral hygiene routine. Typically, manufacturers recommend patients avoid eating for 2 hours to 4 hours after application and avoid brushing until the following day.20
Contraindications of fluoride varnish include ulcerative gingivitis and stomatitis due to the short-term burning sensation that occurs when the product contacts gingival tissue.20 Ulcerative gingivitis and stomatitis may exacerbate these symptoms. Despite the rapid setting time of NaF varnish and the small amount used, ingestion of fluoride is a common risk.20,21 This is more of a concern when applying NaF on primary teeth because young children are more likely to ingest the product during application.
SILVER DIAMINE FLUORIDE
In 2014, the FDA approved silver diamine fluoride (SDF) as a desensitizing agent in adults older than age 21.22 The 38% solution contains 25% silver, 5% fluoride, and 8% ammonia.22 The fluoride promotes remineralization of enamel and dentin surfaces, the silver salts provide an antimicrobial action, and the ammonia helps the solution remain concentrated. SDF is a colorless alkaline liquid, however newer formulas now offer a tinted option, which aids in clinical application.23 SDF at a 38% concentration contains 44,800 ppm fluoride, the highest among all fluoride agents available for use.24 Evidence shows that SDF is an inexpensive and safe treatment option for caries prevention and DH.25–27 Although studies show that SDF significantly reduces symptoms of DH, it is most often used off-label for caries arrest.18,28,29
SDF has an antimicrobial action against cariogenic bacteria such as Streptococcus mutans, Actinomyces naeslundii, and Lactobacillus acidophilus.24 When SDF is applied to the tooth’s surface, it reacts with hydroxyapatite to form silver phosphate and calcium fluoride.30 The fluoride and phosphate ions promote remineralization of enamel and dentin, and the silver ions in SDF can destroy the cell wall of bacteria, inhibit enzyme activities, influence metabolic processes, and inhibit the replication of bacterial DNA. A study by Castillo et al19 found that SDF was effective and safe in treating DH 7 days after application. Evidence demonstrates that 38% SDF is more effective in hardening or arresting caries lesions than 5% NaF varnish, suggesting that SDF may be most effective in treating DH associated with or caused by demineralization and caries.20,21
To apply SDF, the hypersensitive teeth are isolated with cotton rolls, the area is gently dried, and SDF is applied with a disposable microbrush.23 After application, excess product should be wiped away and the treated area should be gently dried. There are no post-application instructions necessary; however, the clinician should refrain from rinsing the treated area. Figure 1 demonstrates the application of SDF with a disposable microbrush.
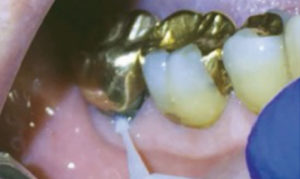
Known contraindications to SDF are silver allergy, presence of stomatitis or ulcerative gingival conditions, and teeth that require pulpal therapy.23 A major disadvantage of SDF is the possibility of staining.22,23 The silver particles in SDF darken active caries lesions, however, SDF does not stain sound enamel. Studies show that black staining only occurs when surfaces have untreated decay.22 It also stains skin and is not easily removed. SDF can permanently stain clothing, floors, and furniture.23 In addition, SDF is known to have an unpleasant metallic taste and can cause gingival irritation.23 This gingival irritation, or burning, causes the tissue to turn white but usually heals within 1 day to 2 days.23 Another disadvantage is SDF’s sensitivity to light.23 Due to this sensitivity, the solution must be kept in a dark container until use.
The use of SDF has been proven effective in managing dental caries and DH; however, its use depends on the dental practitioner’s knowledge, familiarity, and perceptions. A survey evaluating dental hygienists’ familiarity and perceptions of SDF found that 32% of the respondents had never heard of SDF, whereas 22% had heard of SDF but were unsure of how it is used.31 After being informed of SDF’s efficacy, most respondents felt it would be beneficial for patients.31 These findings suggest there is a lack of knowledge surrounding the diagnosis and treatment of DH. Additionally, the research shows that SDF is an underutilized treatment option regarding the management of DH.31
COMPARISON OF COST EFFECTIVENESS
The cost effectiveness of in-office fluoride varnishes varies among populations with different risk levels.20 Schwendicke et al20 found that fluoride varnish costs twice as much and was shown to be slightly more effective than not using fluoride varnish among low-risk populations. For populations at low-risk, fluoride varnish did not significantly reduce caries.20 However, for individuals at high-risk, fluoride varnish was able to reduce caries and the cost-effectiveness was much greater.20 A study evaluating elderly patients in Germany compared the cost-effectiveness of SDF and other preventive agents.32 Results showed that SDF was most cost-effective when compared to fluoride mouthrinses and chlorhexidine among high-risk populations.32
CURRENT EVIDENCE
Although SDF was approved by the FDA as a desensitizing agent, minimal evidence exists to support its use as such.22 Further, no research has been done comparing the efficacy of SDF to NaF varnish in the management of DH. Castillo et al19 found that SDF was effective and safe in treating DH with reductions in sensitivity persisting 7 days after application. Research has shown that NaF varnish is also effective in treating DH, but its effects are short-lived.4,33,34 For arresting caries lesions, 38% SDF is more effective in hardening or arresting caries lesions than 5% NaF varnish.21 Both SDF and NaF are cost-effective; however, research shows that SDF was more cost-effective in the management of root caries when compared to other preventive agents such as fluoride mouthrinses.4,32
CONCLUSION
The painful symptoms associated with DH negatively impact patients’ oral health-related quality of life.2,11,12 Both SDF and NaF varnish are effective in the management of DH symptoms. When considering treatment options for patients experiencing DH, it is important to consider the etiology of symptoms. DH due to demineralization or dental caries may be appropriately treated with SDF, while DH caused by the exposure of dentinal tubules may be more appropriately treated with NaF varnish. Understanding the etiology of an individual’s DH symptoms allows the oral health professional to choose a treatment option tailored to a patient’s individual needs.
REFERENCES
- Vincent, GK, Victoria AV. The next four decades: the older population in the United States: 2010 to 2050. Available at: census.gov/prod/2010pubs/p25-1138.pdf. Accessed May 25, 2019.
- Bekes K, John MT, Schaller H-G, et al. Oral health-related quality of life in patients seeking care for dentin hypersensitivity. J Oral Rehabil. 2009;36:45–51.
- Canadian Advisory Board on Dentin Hypersensitivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc. 2003;69:221–226.
- Osmari D, Fraga S, de Oliveira Ferreira AC, et al. In-office treatments for dentin hypersensitivity: a randomized split-mouth clinical trial. Oral Health Prev Dent. 2018;16:125–130.
- Willershausen I, Schulte D, Azaripour A, et al. Penetration potential of a silver diamine fluoride solution on dentin surfaces: an ex vivo study. Clin Lab. 2015;61:1695–1701.
- Ritter AV, Shugars DA, Bader JD. Root caries risk indicators: a systematic review of risk models. Community Dent Oral Epidemiol. 2010;38:383–397.
- Griffin SO, Griffin PM, Swann JL, Zlobin N. Estimating ratesof new root caries in older adults. J Dent Res. 2004;83:634–638.
- Davari A, Ataei E, Assarzadeh H. Dentin hypersensitivity: etiology, diagnosis and treatment; a literature review. J Dent Shiraz Iran. 2013;14:136–145.
- Haneet RK, Vandana LK. Prevalence of dentinal hypersensitivity and study of associated factors: a cross-sectional study based on the general dental population of Davangere, Karnataka, India. Int Dent J. 2016;66:49–57.
- Bekes K, Hirsch C. What is known about the influence of dentine hypersensitivity on oral health-related quality of life. Clin Oral Invest. 2013;17(Suppl 1):S45–S51.
- Douglas-de-Oliveira DW, Vitor GP, Silveira JO, et al. Effect of dentin hypersensitivity treatment on oral health related quality of life – A systematic review and meta-analysis. J Dent. 2018;71:1–8.
- Petersson LG. The role of fluoride in the preventive management of dentin hypersensitivity and root caries. Clin Oral Investig. 2013;17(Suppl 1):S63–S71.
- American Dental Association Council on Scientific Affairs. Professionally applied topical fluoride: evidence-based clinical recommendations. J Am Dent Assoc 2006;137:1151–1159.
- Clark D, Levin L. Tooth hypersensitivity treatment trends among dental professionals. Quintessence Int. 2018;49:147–151.
- Ritter AV, de L Dias W, Miguez P, et al. Treating cervical dentin hypersensitivity with fluoride varnish: a randomized clinical study. J Am Dent Assoc 2006;137:1013–1020.
- Ipci SD, Cakar G, Kuru B, et al. Clinical evaluation of lasers and sodium fluoride gel in the treatment of dentine hypersensitivity. Photomed Laser Surg. 2009;27:85–91.
- Al-Sabbagh M, Brown A, Thomas MV. In-office treatment of dentinal hypersensitivity. Dent Clin North Am. 2009;53:47–60.
- Orsini G, Procaccini M, Manzoli L, et al. A double-blind randomized-controlled trial comparing the desensitizing efficacy of a new dentifrice containing carbonate/hydroxyapatite nanocrystals and a sodium fluoride/potassium nitrate dentifrice. J Clin Periodontol. 2010;37:510–517.
- Castillo JL, Rivera S, Aparicio T, et al. The short-term effects of diammine silver fluoride on tooth sensitivity: a randomized controlled trial. J Dent Res. 2011;90:203–208.
- Schwendicke F, Splieth CH, Thomson WM, et al. Cost-effectiveness of caries-preventive fluoride varnish applications in clinic settings among patients of low, moderate and high risk. Community Dent Oral Epidemiol. 2018;46:8–16.
- Beltrán-Aguilar ED, Goldstein JW, Lockwood SA. Fluoride varnishes: a review of their clinical use, cariostatic mechanism, efficacy and safety. J Am Dent Assoc. 2000;131:589–596.
- Mei ML, Lo EC-M, Chu C-H. Clinical use of silver diamine fluoride in dental treatment. Compend Contin Educ Dent. 2016;37:93–98.
- Zhao IS, Gao SS, Hiraishi N, et al. Mechanisms of silver diamine fluoride on arresting caries: a literature review. Int Dent J. 2018;68:67–76.
- Zhi QH, Lo ECM, Lin HC. Randomized clinical trial on effectiveness of silver diamine fluoride and glass ionomer in arresting dentine caries in preschool children. J Dent. 2012;40:962–967.
- Caglar E. Efficacy of silver diamine fluoride for caries reduction in primary teeth and first permanent molars of schoolchildren: 36-month clinical trial. J Dent Res. 2007;86:95.
- Yee R, Holmgren C, Mulder J, et al. Efficacy of silver diamine fluoride for arresting caries treatment. J Dent Res. 2009;88:644–647.
- Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012;87:982–990.
- Craig GG, Knight GM, McIntyre JM. Clinical evaluation of diamine silver fluoride/potassium iodide as a dentine desensitizing agent. A pilot study. Aust Dent J. 2012;57:308–311.
- Olusile AO, Bamise CT, Oginni AO, et al. Short-term clinical evaluation of four desensitizing agents. J Contemp Dent Pract. 2008;9:22–29.
- Chu CH, Lo ECM, Lin HC. Effectiveness of silver diamine fluoride and sodium fluoride varnish in arresting dentin caries in Chinese pre-school children. J Dent Res. 2002;81:767–770.
- Chhokar SK, Laughter L, Rowe DJ. Perceptions of registered dental hygienists in alternative practice regarding silver diamine fluoride. J Dent Hyg. 2017;91:53–60.
- Schwendicke F, Göstemeyer G. Cost-effectiveness of root caries preventive treatments. J Dent. 2017;56:58–64.
- Winston AE, Charig AJ, Thong S, et al. Mechanism of action of a desensitizing fluoride toothpaste delivering calcium and phosphate ingredients in the treatment of dental hypersensitivity. Part III: Prevention of dye penetration through dentin vs a calcium- and phosphate-free control. Compend Contin Educ Dent. 2010;31:46–52.
- Hu S, Meyer B, Duggal M. A silver renaissance in dentistry. J Eur Acad Paediatr Dent. 2018;19:221–227.
From Dimensions of Dental Hygiene. June 2019;17(6):48–51.



