
Ensuring Safe Dental Unit Waterlines
Learn about the critical aspects of maintaining dental unit water quality, preventing biofilm formation, and reducing infection risks in dental practices.
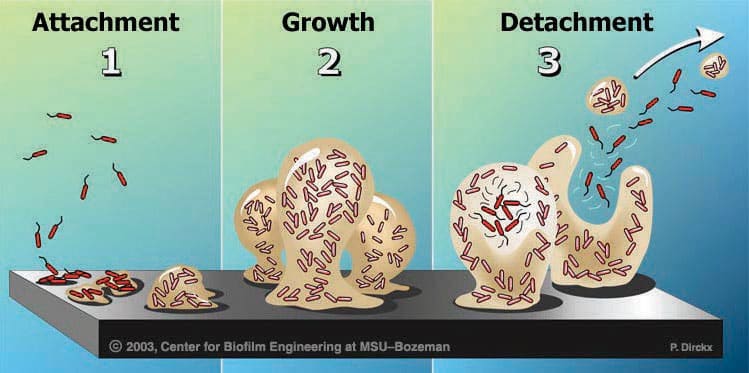
Biofilms are a collection of microorganisms firmly attached to a moist surface by a slimy matrix that can survive in a variety of environments. Biofilms are found in nature, industry, and healthcare settings — including dental unit waterlines (DUWLs). Two forms of bacteria are related to biofilm formation: planktonic (single free-floating) and attached communities (groups or clusters). Initially, planktonic bacterial cell walls adhere to surfaces using a polymer (glycocalyx). More bacteria begin to proliferate and colonize, giving the complex microbial colony a protective slimy layer (Figure 1).
DUWLs are ideal environments for biofilm formation because they are made of narrow tubing material, have low flow rates, are prone to stagnation in the lines, and may retract oral fluids.1,2 DUWLs include tubing to the air/water syringe and handpiece lines. Microorganisms thrive, colonize, and proliferate on the inner surface of waterline tubing—creating biofilms. Outgoing water from the dental unit may be contaminated if planktonic bacteria or pieces of biofilm break off and enter the water stream.2 The United States Food and Drug Administration (FDA) considers dental units as Class I regulated medical devices. As such, DUWLs must be “routinely cleaned and disinfected.”3
Clinical Implications
Numerous waterborne microorganisms have been identified in DUWLs, but most are not pathogenic to patients with healthy immune systems.2 Unfortunately, some of these species, such as Pseudomonas, Legionella and Mycobacterium, can be harmful or deadly to immunocompromised patients. In 2011, an otherwise healthy 82-year-old woman died from irreversible septic shock after acquiring Legionnaire disease (Legionella pneumophila) from dental unit water used during treatment.4 As more immunocompromised patients are treated in general dental practices, reducing exposure risk to harmful pathogens is important.
The US Centers for Disease Control and Prevention (CDC) issued an official health alert on October 31, 2022 emphasizing “the importance of following existing recommendations for maintaining and monitoring dental waterlines” after two outbreaks of nontuberculosis Mycobacteria (NTM) were discovered in large pediatric dental clinics in California and Georgia.5 These outbreaks were a result of contaminated DUWLs that caused serious infections in young children who had undergone pulpotomies. These clinics were not treating or monitoring their DUWLs.
Guidelines for Dental Unit Water Quality
The Environmental Protection Agency (EPA), American Public Health Association, and American Water Works Association set standards for safe drinking water at < 500 CFU/mL. The 2003 CDC Guidelines for Infection Control in Dental Health-Care Settings state that bacterial counts in outgoing dental unit water (for nonsurgical procedures) should be “as low as reasonably achievable” and must minimally meet EPA drinking water standard of < 500 CFU/mL.2
Additionally, the 2016 CDC Summary of Infection Prevention Practices in Dental Settings states, “All dental units should use systems that treat water to meet drinking water standards (ie, ≤ 500 CFU/mL of heterotrophic water bacteria). Use water that meets EPA regulatory standards for drinking water (≤ 500 CFU/mL of heterotrophic water bacteria) for routine dental treatment output water.”6
The Organization for Safety, Asepsis and Prevention (OSAP) white paper recommends stakeholders meet or exceed the recommendations set by CDC to achieve the lowest level of contamination.7 The < 500 CFU/mL is an “action limit” intended to identify failure and prompt action to improve DUWL quality. A well-maintained DUWL system should test in the 0 to 100 CFU/mL range indicating biofilm control. It is impossible to completely eliminate biofilms in DUWL, but keeping the CFU/mL count as slow as possible is the goal.2,7 A variety of devices are available to help dental practices meet these recommendations.
Water Quality Basics
Education, scheduled maintenance, standard operating procedures (written step-by-step instructions), and recordkeeping help to ensure that DUWLs meet EPA safe drinking water standards.2,8 Without these measures, biofilms will continue to proliferate and may cause serious harm to patients. The following recommendations from the CDC and OSAP are designed to ensure DUWL safety.2,7
- Consult with the dental unit manufacturer to obtain guidance on DUWL products and protocols that can be used with the unit to ensure proper maintenance scheduling.
-
![]()
Figure 2. To ensure the safety of dental unit waterlines, a self-contained water reservoir with added cleaning agents designed to isolate water from municipal supplies must be used. Use commercially available devices, products, and procedures, including self-contained water reservoirs (Figure 2, page 14) with added cleaning agents designed to isolate water from municipal supplies; chemical or antimicrobial treatments designed to inactivate biofilms; slow-release cartridges to prevent biofilm attachment; inline microfilters; or a combination of these. Products that carry a disinfectant claim must be EPA-registered.2,7 Devices for waterline treatment that are attached to the dental unit must be registered with the FDA as medical devices.7
- Use safe-source water (< 500 CFU/mL) which includes, tap, distilled, or sterile water.7
- Maintain self-contained water reservoirs according to the manufacturer’s instructions to prevent contamination. Self-contained water alone will not eradicate biofilm formation in the DUWL, so controlling the quality (either periodically or continuously) via devices, products, or procedures is necessary.2,7
- Flush all DUWLs for 20 to 30 seconds between patients to remove any possible contamination from the previous patient. Most dental units are equipped with anti-retraction valves to prevent “suck-back” of contaminated fluid. Even with these valves, flushing is still recommended. There is no longer a need to flush DUWLs for 1 to 2 minutes at the beginning of the day, because firmly adherent biofilms are not dislodged by flushing alone, therefore, flushing for 20 to 30 seconds is adequate to clear stagnant lines. Flushing alone does not qualify as good quality control.2,7
- Retrofit dental units that are more than 20 years old with anti-retraction valves.2,7 Use the shortest available tubing from the water source to the handpiece to reduce the surface area for biofilm formation. Remove additional unnecessary tubing pathways known as “dead legs” that can harbor biofilm.
- If municipal water is used, install a point-of-use filter between the DUWL tubing and the instrument, or retrofit the unit for a self-contained water system.2
Water Options and Source Water
Options for water management include independent water bottle reservoirs, chemical treatments, water treatment devices, antimicrobial tubing, or a combination of these. Independent water bottle reservoirs are an economical option, but are useless without some sort of chemical treatment such as tablets, liquids, and cartridges.7–9 Independent reservoirs — or water-bottle systems — alone are not sufficient. Consult with the dental unit manufacturer for appropriate water maintenance methods and recommendations for monitoring water quality.6 Water can also be treated with a germicidal in-line filter attached to the incoming municipal water.7 Always seek guidance from the dental unit manufacturer to check for compatibility.
Appropriate source water must be used with independent water bottle reservoir systems.2,7 Quality source water can include water from reverse osmosis systems, distillation, deionization, germicidal ultraviolet light, tap, and filtration. No matter how clean the source water is, recurrent shock treatment or continuous germicidal treatment is necessary to reduce biofilm formation.7
Monitoring Dental Water Quality
To ensure safe water standards, all dental professionals should be educated about dental unit water quality, biofilm, water treatment, and dental unit maintenance including monitoring of water quality.2,7 Monitoring (or testing) water quality helps determine the safety of the outgoing dental unit water and compliance with monitoring schedules. Dental unit manufacturers should be consulted for proper instructions to maintain safe water standards.
Monitoring of dental unit water quality can be achieved by one of two methods: commercial water testing products/kits used in the office (chairside paddle tests) or a commercial water testing laboratory service.2,7 Commercial testing laboratories use a gold standard test to determine exact bacterial CFU counts. In-office chairside water testing kits are screening tools that may provide consistent results. Research findings on the efficacy of in-office testing products are mixed and more study is needed.7 Chairside tests are easy to use, which can increase compliance, but they may not provide consistent results when compared to laboratory testing.
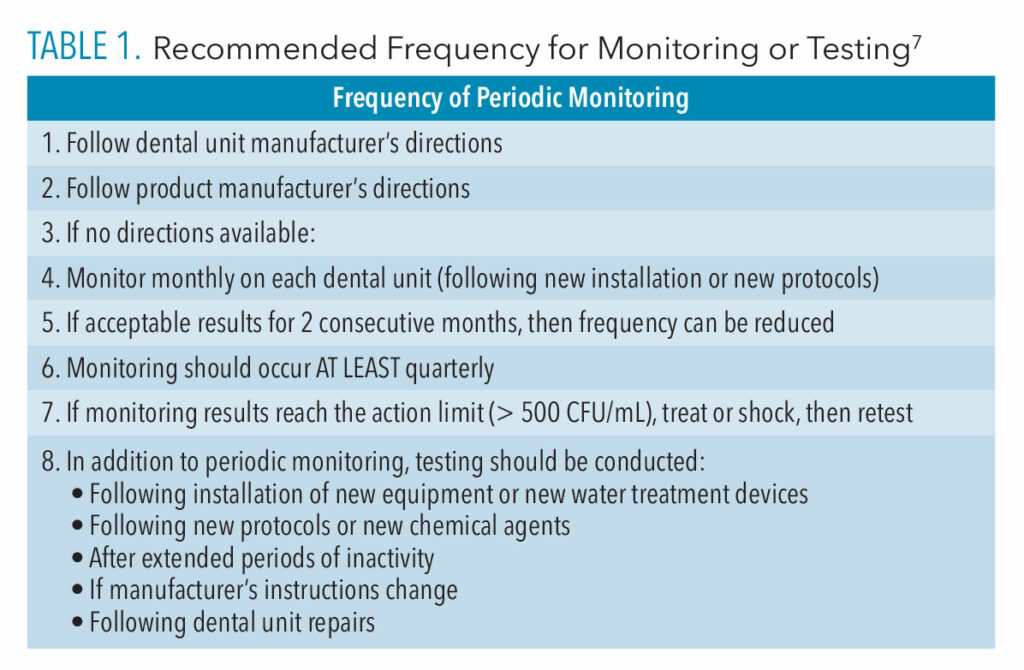 Compliance with monitoring protocols is important, no matter which testing method is used. The 2003 and 2016 CDC guidelines do not outline specific procedures or maintenance schedules for monitoring dental unit water. However, the OSAP white paper provides more specific recommendations for frequency of testing.7 OSAP recommends testing water before any chemical treatments are administered, when new products or protocols are used, and if a new employee is taking on the water testing responsibility.7 Table 1 outlines OSAP’s recommended frequency for monitoring.
Compliance with monitoring protocols is important, no matter which testing method is used. The 2003 and 2016 CDC guidelines do not outline specific procedures or maintenance schedules for monitoring dental unit water. However, the OSAP white paper provides more specific recommendations for frequency of testing.7 OSAP recommends testing water before any chemical treatments are administered, when new products or protocols are used, and if a new employee is taking on the water testing responsibility.7 Table 1 outlines OSAP’s recommended frequency for monitoring.
Effective water monitoring is based on a variety of factors. Proper infection control standards should be followed to avoid contamination when collecting samples. All manufacturer instructions should be followed, and records of monitoring results need to be kept for each unit in the practice. Prudent record keeping of DUWL monitoring should include sampling information such as the source, date, time of collection, identity of person collecting the sample, method of analysis (chairside test or lab test), test results, remediation efforts for failed tests and any follow up results, and location of monitoring log (record keeping document).7
Maintenance of Dental Unit Waterlines
Once the quality of the dental unit water is determined through testing, initial, periodic, or continuous chemical “shock” treatments are recommended to enhance the performance of waterline treatment products and to reduce or eliminate biofilm.7 Some products are used initially or periodically to reduce or eliminate biofilm, while others provide continuous chemicals in the waterline to deter biofilm formation. Periodic or continuous products include tablets, liquids, continuous release cartridges, and centralized filtration systems.8
Periodic treatments require purging of the waterline, adding chemical additives to the water reservoir, filling the lines for the recommended time period, and then flushing. Continuous chemical treatments with automated metering devices release low levels of chemicals into the treatment water to control biofilm — lowering bacterial counts in the water.7 Some products require a combination of periodic and continuous shocking.
Shocking DUWLs consists of a chemical treatment to reduce or eliminate biofilms. A variety of shock treatments are available. Always consult the manufacturer’s instructions. After shock treatments are administered, routine maintenance procedures and schedules should be followed according to manufacturer instructions. Depending on the product or unit, these guidelines typically include daily, weekly, quarterly, and/or annual protocols. The dental unit manufacturer should provide guidance on the maintenance schedule associated with their product.
![]() Surgical Procedures
Surgical Procedures
Oral surgical procedures put patients at increased infection risk because of the invasive nature of these procedures, so sterile solutions must be used for irrigation and cooling. Traditional dental units cannot always deliver sterile water due to biofilm formation in DUWLs, so sterile irrigation solutions are recommended for oral surgical procedures. Sterile saline or sterile water should be used as a coolant/irrigant when performing oral surgical procedures. Only devices specifically designed for delivering sterile irrigating fluids (eg, bulb syringe, single-use disposable products, and sterilizable tubing) should be implemented.2,7-10
The American Academy of Pediatric Dentistry (AAPD) supports the use of sterile solutions for irrigation and cooling, especially in light of the numerous NTM outbreaks in pediatric dental clinics. The AAPD encourages development and implementation of DUWL policies and protocols.10 Following the guidance of CDC, OSAP, and the AAPD should help to reduce or eliminate further NTB outbreaks and increase compliance among practitioners.
Conclusion
Prudent infection control practices are vital in providing safe patient care. Monitoring and maintenance of dental unit water quality is an essential part of routine infection control practices. Table 2 outlines practices the dos and don’ts of DUWL maintenance.11 All dental professional should be trained and calibrated using standard operating procedures to ensure that dental equipment is maintained properly to produce acceptable water quality standards.
References
- Organization for Safety, Asepsis, and Prevention. Dental Unit Waterlines Resources. Available at: osap.o/g/topics-dental-unit-waterlines-duwl. Accessed September 29, 2023.
- Kohn WG, Collins AS, Cleveland JL, et al. Guidelines for infection control in dental healthcare settings MMWR Recomm Rep. 2003;52(RR-17):1-61.
- United States Food and Drug Administration. Dental Unit Waterlines. Available at: fda.gov/medical-devices/dental-devices/dental-unit-waterlines. Accessed September 29, 2023.
- Ricci ML, Fontana S, Pinci F, et al. Pneumonia associated with a dental unit waterline. Lancet. 2012;379:684.
- United States Centers for Disease Control and Prevention. Outbreaks of Nontuberculosis Mycobacteria Infections Highlight Importance of Managing and Monitoring Dental Waterlines. Available at: emergency.cdc.gov/han/떖/han00478.asp Accessed September 29, 2023.
- US Centers for Disease Control and Prevention. Dental Unit Water Quality: Summary of Infection Control Practices in Dental Settings. Available at: cdc.gov/oralhealth/infectioncontrol/summary-infection-prevention-practices/dental-unit-water-quality.html. Accessed September 29, 2023.
- Mills S, Porteous N, Zawada J. Dental Unit Water quality: organization for safety, asepsis and prevention white paper and recommendations–2018. Journal of Dental Infection Control and Safety. 2018;1(1):1-27.
- US Centers for Disease Control and Prevention. Dental Unit Water Quality. Available at: cdc.gov/oralhealth/infectioncontrol/faqs/dental-unit-water-quality.html#print Accessed September 29, 2023.
- US Centers for Disease Control and Prevention. Summary of Infection Prevention Practices in Dental Settings. Basic Expectations for Safe Care. Available at: cdc.gov/oralhealth/infectioncontrol/pdf/safe-care2.pdf. Accessed September 29, 2023.
- American Academy of Pediatric Dentistry. Policy on Infection Control. Available at: aapd.org/research/oral-health-policies–recommendations/infection-control. Accessed September 29, 2023.
- American Dental Association. Managing the Regulatory Environment ADA’s Guidelines for Practice Success. Do’s and Don’ts for Maintaining Dental Unit Water Lines. Available at: ada.org/-/media/project/ada-organization/ada/ada-org/files/publications/guidelines-for-practice-success/gps-regulatory/maintaining-dental-unit-water-lines.pdf?rev=f3bbd50e359449fdb90be11909c7bfa7&hash=B3DA2BAB2609A066D3B784FB41FBD1FF. Accessed September 29, 2023.
From Dimensions in Dental Hygiene. October 2023; 21(9):10-15



