
Detecting Hereditary Hemorrhagic Telangiectasia
Dental hygienists can improve health outcomes by recognizing the first symptoms of this genetic disorder.
This course was published in the April 2012 issue and expires April 2015. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Identify the symptoms of hereditary hemorrhagic telangiectasia (HHT).
- Discuss the diagnosis and treatment of HHT.
- Explain the dental implications of treating patients with HHT.
It has been 12 years since the release of “Oral Health in America: a Report of the Surgeon General,” which stressed the relationship between oral and systemic health.1 As a result, more dental professionals are collaborating with other disciplines to address their patients’ systemic health. Dental hygienists should be on the front lines of helping patients manage their general health in addition to promoting good oral health. Frequent dental visits provide an opportunity for dental hygienists to systematically follow patients’ medical and dental histories, which may help them recognize developing conditions, such as hereditary hemorrhagic telangiectasia (HHT).
HHT is an extremely underdiagnosed condition,2–8 and without accurate diagnosis and treatment, it can lead to serious morbidity and mortality (Table 1).3 Unfortunately, HHT has not been reported much in the dental or dermatologic literature. Most people with HHT eventually present with telangiectasias (appearing as red to purplish dots) on the skin of the hands, face, and mouth—areas clearly noticeable during the dental appointment. Dental hygienists can make a difference by noting these symptoms and referring patients for accurate diagnosis and treatment before serious health consequences ensue.
Etiology
 HHT is a hereditary autosomal dominant disease, thus, children of a parent with HHT have a 50% chance of contracting the disorder. The prevalence of HHT is approximately one in 5,000 North Americans, affecting 70,000 people in the United States and more than 1.2 million people worldwide.7,9 Recent advances in molecular genetics have provided greater insight into this genetic disorder, but its variability, even among members of the same family, make diagnosis and management problematic.
HHT is a hereditary autosomal dominant disease, thus, children of a parent with HHT have a 50% chance of contracting the disorder. The prevalence of HHT is approximately one in 5,000 North Americans, affecting 70,000 people in the United States and more than 1.2 million people worldwide.7,9 Recent advances in molecular genetics have provided greater insight into this genetic disorder, but its variability, even among members of the same family, make diagnosis and management problematic.
HHT is characterized by alterations in the growth factor receptors of endothelial cells in blood vessels.8 A focal dilation of postcapillary venules, which continue to grow and connect with dilated arterioles, is usually the first detectable lesion. Over time, as the lesion begins to enlarge, the capillaries completely disappear and produce a complete venule and arteriole connection. The junction between the artery and vein is thin, producing dilation in the vessel wall, which is called a telangiectasia.
In a fully developed telangiectasia, the venule and/or arteriole is very dilated and convoluted, and presents superficially on the skin or mucosal surfaces as a red/purplish dot. A larger vessel dilation, such as those found in some internal organs, are called arteriovenous malformations (AVMs). Telangiectasias and AVMs are primarily asymptomatic but prone to rupture, producing bleeding episodes that can have serious consequences depending on the location—especially in the case of AVMs.8
Symptoms
Symptoms of HHT vary greatly, but the most common areas for telangiectasias are the skin and nasal and oropharyngeal cavities. The earliest and most common symptom is recurrent nosebleeds, which are present in more than 90% of affected patients.2–9 Nosebleeds begin in most patients by adolescence,3 and can range in severity from harmless to so severe that blood transfusion is required.
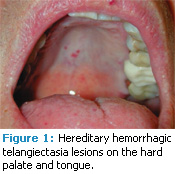
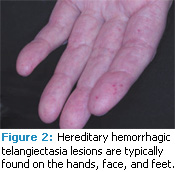
Telangiectasias of the skin and buccal mucosa occur in approximately 75% of patients with HHT, are usually visible by age 30, and increase in size and number with age.4 Skin lesions primarily appear on the hands, face, and feet.8 Intraorally, they can present on the tongue, labial/buccal mucosa, palate, and gingiva, although any oral mucosae can be affected.10–12
Clinically, the telangiectatic areas appear as red to blue macules and/or papules, depending on the area affected. Skin lesions tend to be macular red spots from 1 mm to more than 7 mm in size, while intraoral lesions can be macular or papular with the same variation in size. Oral lesions are often described as “pinpoint-sized” or “pea-sized.”3 The lesions may appear as petechiae, but they all blanch upon pressure. They are asymptomatic but prone to rupture and hemorrhage (Figure 1 and Figure 2).
Clinically, the telangiectatic areas appear as red to blue macules and/or papules, depending on the area affected. Skin lesions tend to be macular red spots from 1 mm to more than 7 mm in size, while intraoral lesions can be macular or papular with the same variation in size. Oral lesions are often described as “pinpoint-sized” or “pea-sized.”3 The lesions may appear as petechiae, but they all blanch upon pressure. They are asymptomatic but prone to rupture and hemorrhage (Figure 1 and Figure 2).
AVMs in the organs can be congenital or develop over time, and they may be asymptomatic.7 The most frequently affected internal organs include the lungs, brain, gastrointestinal tract, and liver.2–9 Pulmonary AVMs (PAVM) are the most common, appearing in roughly 30% to 40% of patients with HHT. PAVMs can lead to hypoxemia, are prone to hemorrhage if not treated, and can cause serious health problems because emboli can travel from the lungs and lodge in the brain. Cerebral AVMs are the least common (10% to 15%) and can result in headaches, seizures, and ischemia in the case of a hemorrhage. Gastrointestinal bleeding from telangiectasia can result in melena and iron deficiency anemia.9,11 Frequent nosebleeds can cause iron deficiency anemia, as well. AVMs in the liver are mostly asymptomatic and produce few problems for the patient.13
Table 1. Hereditary Hemorrhagic Telangiectasia At a Glance
- Hereditary hemorrhagic telangiectasia (HHT), also known as Osler-Weber-Rendu Syndrome, is a multi-system vascular dysplasia.
- Approximately 1.2 million people worldwide have HHT, making the disorder uncommon, but not rare.
- Telangiectasias and arteriovenous malforma – tions (AVMs) are the characteristic lesions.
- Symptoms range from mild to severe.
- Physicians frequently miss the diagnosis in affected individuals.
- Most commonly affected organs are nose, lungs, gastrointestinal tract, brain, and spine.
- HHT is an autosomal dominant genetic disorder. Denovo mutations are rare. A targeted family history shows almost all cases to be familial.
- HHT is heterogenic with defects in at least three genes.
- It is extremely rare for one individual to present with all manifestations of HHT.
- The severity of a nosebleed does not predict the likelihood of internal (ie, brain and lung) manifestations.
- Severity of the disorder varies tremendously, even between close relatives.
- Treatments are available for all manifestations of HHT.
- Men and women are affected by HHT in equal numbers.
- The HHT Foundation International recommends that patients receive an assessment at a specialized HHT center at least once as early in life as possible. Diagnosis and treatment for HHT is complex and has changed significantly in the past decade.
Reprinted with permission from HHT Foundation International
Diagnosis and Treatment
In 1999, the Curacao criteria were developed for diagnosing HHT (Table 2).2 Three of the four criteria must be present for a definite diagnosis of HHT. The disease is suspected if two criteria are met, and unlikely if only one is present.2,9 Screening should include contrast echocardiography of the lungs and magnetic resonance image (MRI) of the brain. Genetic testing can be performed as well, and is preferred for families with a history of HHT. Most, however, are unaware that they carry the gene for the disease.
HHT is often unrecognized by health care providers. The HHT Foundation International estimates that nine out of 10 people with HHT go undiagnosed.9 Among people with undiagnosed HHT, 20% are either disabled or die due to lack of recognition of the disease and subsequent care.9 The literature contains several reports of preventable deaths due to HHT among adults, adolescents, and children who went undiagnosed. 2,9,14 If the condition is diagnosed, AVM complications can be prevented.
Table 2. Curacao Criteria for Diagnosing Hereditary Hemorrhagic Telangiectasia
- Spontaneous and recurrent nosebleeds
- Multiple telangiectasias at characteristic sites
- Internal arteriovenous malformations
- Family history
A variety of treatments are available for HHT, depending on the size and location of the telangiectasias or AVMs. Laser coagulation therapy works well for nasal telangiectasias that cause nosebleeds. Therapeutic drug sprays are also being studied as possible treatments. Skin lesions can be treated with a laser as well. Lung lesions are addressed by embolization (blocking off an artery). Brain AVMs are sometimes treated with more than one modality, depending on the size, structure, and location of the abnormal vessels. Gastrointestinal bleeding is usually only treated if it causes anemia, with iron therapy providing the first step in treatment.9
Dental Implications
The dental hygienist is in an optimal position to recognize the symptoms of HHT. Medical/dental history forms should be updated at every recare visit. Health history forms should include questions about frequent nosebleeds in both patients and their family members. During patient assessment, any telangiectasias occurring intraorally; extraorally; or on the head, neck, or hands should be identified and documented.
Unfortunately, symptoms of other oral health problems may mimic HHT oral lesions. Several lesions in the oral cavity, such as palatal petechiae, which can have multiple etiologies, appear similar to HHT oral lesions. The appearance of telangiectasias on the skin can also be very subtle, and they may resemble cherry angiomas.15 If HHT is suspected, the patient’s health history should be reviewed very carefully, with special attention paid to familial nosebleeds, the addition of new lesions, and changes to any lesions noted during the last visit.
When treating patients with diagnosed HHT in the dental office, stringent assessment procedures and appropriate documentation should be implemented at each recare visit. Many patients with HHT need antibiotic prophylaxis before undergoing any dental procedure that may produce bacteremia, such as oral debridement.2,3,8,10 This is primarily due to problems from PAVMs, which lack capillaries to filter the blood as it passes through the lungs, allowing bacteria to travel to the brain—potentially producing a cerebral abscess. If a patient with HHT has not had the appropriate diagnostics test for pulmonary AVMs, dental treatment should be delayed until testing is accomplished.3,10,16–18 Researchers have reported cases of brain abscesses among patients with HHT who had teeth extracted without antibiotic premedication.17,18 Premedication before dental procedures is not necessary for patients who have AVMs in other organs, but mandatory for those with AVMs in the lungs. During treatment, patients with HHT should remain upright to reduce the risk of nasal bleeding. Anti-inflammatory drugs, such as ibuprofen, naproxen, and aspirin, should be avoided due to the increased risk of bleeding.3,7 Oxygen should be readily available, and blood pressure should be monitored before and after treatment.18
The purview of dental hygienists is expanding to include risk assessment and systemic health promotion. The ability to recognize symptoms and safely treat patients with HHT supports the role of the dental hygienist as a knowledgeable and effective health care provider. For more information about HHT, visit www.hht.org.
Case Study
 A 57-year-old male patient presented with a long history of recurrent nosebleeds—approximately once per day, lasting 6 minutes to 15 minutes, which began in his mid-20s. He also experienced bleeding gums while brushing his teeth several times per week. Intraorally, telangiectasias were present on the tongue and hard palate. Skin lesions were noted on the palmar surfaces of his hands, as well as on the left check and along the pinna of his left ear.
A 57-year-old male patient presented with a long history of recurrent nosebleeds—approximately once per day, lasting 6 minutes to 15 minutes, which began in his mid-20s. He also experienced bleeding gums while brushing his teeth several times per week. Intraorally, telangiectasias were present on the tongue and hard palate. Skin lesions were noted on the palmar surfaces of his hands, as well as on the left check and along the pinna of his left ear.
After seeking care from multiple specialists, he was diagnosed with HHT through assessment with the Curacao criteria. Additional examination revealed small AVMs of the pancreas and spleen, which were considered innocuous. An MRI of the brain showed nothing abnormal. No gross PAVMs were discovered, but there was evidence of intrapulmonary shunting, a common side effect of HHT in which a region of the lungs is perfused with little or no filter capacity. This finding warranted the use of antibiotics prior to certain procedures, especially dental treatments, as per the American Heart Association endocarditis prophylaxis guidelines.19 He was encouraged to repeat his chest computed tomography scan again in 5 years in case of focal macroscopic development of pulmonary AVMs. He was also advised to undergo regular surveillance for the development of iron deficiency anemia as an indicator for the onset of significant occult bleeding due to gastrointestinal telangiectasias. This particular patient had seen several physicians and oral surgeons before he was finally diagnosed with HHT.
Patient information provided by Michelle Atkins, RDH.
Acknowledgement:
The author would like to thank Michelle Atkins, RDH, and Kelsey Evans, RDH, BS, for their contributions to this manuscript.
PHOTO CREDITS:
FIGURES COURTESY OF HHT FOUNDATION INTERNATIONAL
REFERENCES
- US Department of Health and Human Services. Oral Health in America: A Report of the Surgeon General. Available at: www.surgeon general.gov/library/oralhealth. Accessed March 7, 2012.
- Olitsky SE. Hereditary Hemorrhagic Telangiectasia: diagnosis and management. Am Fam Physician. 2010;82:785–790.
- Christensen GJ. Nosebleeds may mean something much more serious: an introduction to HHT. J Am Dent Assoc. 1998;129:635–637.
- Begbie ME, Wallace GF, Shovlin CL. Hereditary haemorrhagic telangiectasia (Osler-Weber-Rendu syndrome): a view from the 21st century. Postgrad Med J. 2003;79:18–24.
- Sabba C. A rare and misdiagnosed bleeding disorder: hereditary hemorrhagic telangiectasia. J Thromb Haemost. 2005;3,2201–2210.
- Giordano P, Nigro A, Lenato GM, et al. Screening for children from families with Rendu- Osler-Weber disease: from geneticist to clinician. J Thromb Haemost. 2006;4:1237–1245.
- Sekarski LA, Spangenberg LA. Hereditary hemorrhagic telangiectasia: children need screening too. Pediatr Nurs. 2011;37:163–168.
- Sharathkumar AA, Shapiro A. Hereditary hemorrhagic telangiectasia. Hemophilia. 2008;14:1269–1280.
- Hereditary Hemorrhagic Telangiectasia (HHT) Foundation International. Medical summary. Available at: www.hht.org/medical-scientific/medical-summary. Accessed March 6, 2012.
- Edwards PC, McVaney T. External cervical root resorption involving multiple maxillary teeth in a patient with hereditary hemorrhagic telangiectasia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:585–591.
- Neville BW, Damm DD, Allen CM, Bouquot JE. Epithelial Pathology. In: Dolan J, ed. Oral and Maxillofacial Pathology. 3rd ed. St. Louis: Saunders; 2009:754–755.
- Regezi JA, Sciubba JJ, Jordan RC. In: Dolan J, ed. Oral Pathology: Clinical Pathologic Correlations. 5th ed. St. Louis: Saunders; 2012:117.
- Song X, Chen H, Chen Y, et al. Individualized management of hepatic diseases in hereditary hemorrhagic telangiectasia. Am Surg. 2011; 77:281–285.
- Byard RW, Schliebs J, Koszyca BA. Osler-Weber- Rendu syndrome—pathological manifestations and autopsy considerations. J Forensic Sci. 2001;46:698–701.
- Folz BJ, Lippert BM, Wollstein AC, Tennie J, Happle R, Werner JA. Mucocutaneous telangi ectasia of the head and neck in individuals with hereditary hemorrhagic telangiectasia—analysis of distribution and symptoms. Eur J Dermatol. 2004;14:407–411.
- Shovlin C, Bamford K, Wray D. Post-NICE 2008: Antibiotic prophylaxis prior to dental procedures for patients with pulmonary arteriovenous malformations (PAVMs) and hereditary haemorrhagic telangiectasia. Br Dent J. 2008;205:531–533.
- Corre P, Perret C, Isidor B, Khonsari RH. A brain abscess following dental extractions in a patient with hereditary hemorrhagic telangiectasia. Br J Oral Maxillofac Surg. 2010;49:9–11.
- da Silva Santos PS, Fernandes KS, Magalhaes MH. Osler-Weber-Rendu Syndrome—dental implications. J Can Dent Assoc. 2009;75:527–530.
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116:1736–1754.
From Dimensions of Dental Hygiene. April 2012; 10(4): 48-51.



