 FOTOGENICSTUDIO/ISTOCK/GETTY IMAGES PLUS
FOTOGENICSTUDIO/ISTOCK/GETTY IMAGES PLUS
Update on Dental Restorative Materials
How to safely and effectively care for dental restorations.
This course was published in the January 2019 issue and expires January 2022. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the science of safe finishing and polishing operations.
- Describe the structure and properties of restorative materials that put them at risk.
- Identify precautions for dental hygiene procedures.
Click here to download a summary of restorative materials, microstructures, and generic composites
Dental hygienists routinely deal with prophylaxis of not only teeth, but a vast array of restorative materials. These restorations involve metallic, ceramic, polymeric, and composite materials that are part of the huge armamentarium representing old and new products employed over the past 50 years. More than 1,000 restorative products may be encountered, most of which are not specifically identified in a patient’s record. A dental hygienist needs to be able to recognize various restorative materials and employ the correct treatment protocol. The goals of this review are to summarize the key principles for safe finishing and polishing operations, consider the structure and properties of restorative materials that put them at risk, and identify precautions for dental hygiene procedures.
Metals, ceramics, polymers, and composites are synthetic restorative materials. Metals include amalgam, removable partial denture frameworks, implants, gold, and other casting alloys. Ceramics may be porcelain, porcelain fused-to-metal, porcelain veneers, and high-strength ceramics. Polymers involve infiltrants and polymethyl methacrylate (PMMA) denture base materials. Composites encompass dental composites, glass ionomers, and temporaries. Management of these materials during a dental hygiene appointment requires some understanding of a material’s structure (arrangement, bonding, composition, defects) and properties (physical, chemical, mechanical, biological).1 Clean and smooth surfaces are essential for esthetics, biological health, and long-term resistance to restorative material degradation. This article will focus on identifying unintended effects of cleaning and prevention methods on surfaces.
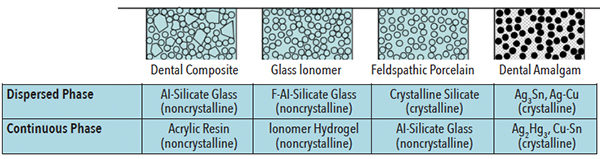
The microstructure of all restorative materials is based on the chemical phases that exist within a material. A simplified view of microstructures is as a continuous and dispersed phase (Figure 1).2 For example, a dental composite with a dispersed phase of silica filler reinforces a continuous phase, which is based on crosslinked polymer and difunctional monomers. Another example is a dental amalgam with dispersed phases of residual crystalline amalgam alloy particles within a continuous phase of crystalline reaction products.
During a routine prophylaxis (plaque, calculus, and stain removal; surface smoothening) or prevention procedures (topical fluoride applications), a restorative material’s surface may be altered. Softer phases may be inadvertently or selectively removed. The dispersed phase is often chemically different and provides reinforcement properties. It may react differently to finishing and polishing. The best results are achieved by using polishing materials that are softer than both the dispersed and continuous phases of the restorative material.
For a variety of reasons, surfaces also may require gentle smoothening (finishing) by leveling of irregularities after setting, reactions after setting, or intraoral wear. Polishing is intended to remove plaque, stain, or corrosion. These abrasion processes are two-body (ie, surface and abrader) or three-body (ie, surface; lubricant, such as saliva or water; and abrader). The most commonly used, the three-body process is the preferred protocol. Cleaning is desired without substantial surface abrasion. Polishing agents must contain materials that are hard enough to remove plaque or stain, but soft enough to not damage surfaces.
Risks from wear or abrasion are relatively easy to rank in terms of a Mohs Hardness Scale (Table 1).2 Hardness of any material is its mechanical resistance to plastic deformation. Mohs scale comparisons involve two materials being rubbed together to see which one is scratched by the other. This scale spans all material hardness, from the softest (talc = 1) to the hardest (diamond = 10). Hardness is 5–6 for enamel, 3–4 for dentin, and 2–3 for cementum. Polishing agents should be softer than enamel or any of the soft phases in a restorative material.
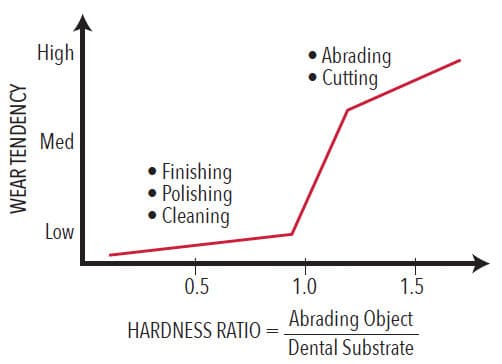
While the primary consideration in polishing involves the hardness of materials, there are other factors, such as type of wear, duration of wear, applied pressure, and size of polishing particles. Larger particles produce greater wear. Smaller particles may erode softer phases. Dentifrices are designed to accomplish the same result as polishing procedures and are subject to the same conditions. Hardness ratios are typically used to summarize the relative likelihood of a potentially abrasive material to produce surface wear (Figure 2).2
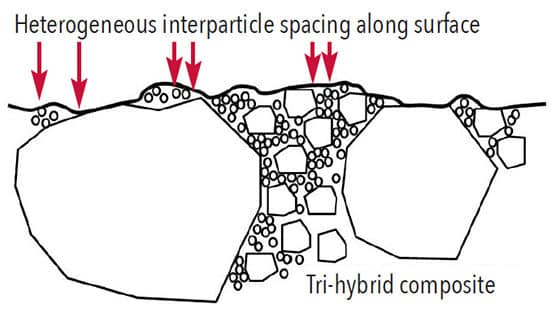
Wear of composites intraorally occurs due to small silica particles (eg, 0.1 µm) within food that abrade the surface by removing softer continuous phases. For restorative composites, this risk is dramatically reduced by high filler particle loading, combining two or three particle sizes for better particle packing, and minimizing average interparticle spaces to less than 0.1 µm.3 Composites wear, but the process is very slow. Longevity for posterior composites equal those of dental amalgams.4,5 Particles in prophylaxis materials must be soft, and have little tendency to abrade the polymer phase.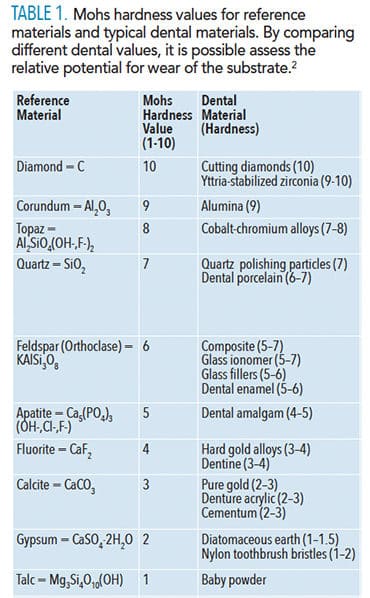
SPECIAL PRECAUTIONS FOR DENTAL HYGIENE
While most smoothening and polishing procedures are straightforward, there are special precautions worthy to note.
Tooth-colored materials (composites, glass ionomers, temporary, or provisional restorations). Without prior knowledge, it is generally difficult to identify a composite vs a glass ionomer restoration. Both are esthetic and have a continuous polymer phase with a dispersed silicate phase. Dental hygienists should be careful not to apply too much pressure during the polishing stage of the prophylaxis or the continuous polymer phase could slowly become abraded. Surface stains are easy to remove. Marginal stains associated with Class I, II, III, and V restorations, as well as veneers, involve discoloration that cannot be removed without damaging the restorative material. Do not aggressively polish at the margins. A variety of composites (macrofill, midifill, minifill, microfill, nanofill, and bi-hybrid or tri-hybrids, and glass ionomer materials) are available, but the various types are sufficiently similar that the same approach should apply.6
Amalgam. Tarnish or electrochemical corrosion products create a darkened or blackened appearance. Removing corrosion products produces a reflective metallic appearance that may be good for cleanliness but does not increase the material’s longevity. Inadvertent dry polishing of an amalgam and/or excessive pressure generates surface heat that easily melts the Ag2Hg3 reaction product (melting point = 127° C)7 within the continual phase—releasing and smearing Hg on the surface. The amalgam looks shiny because of its Hg-rich surface layer, but that smear layer is quickly lost over the next few days, exposing the patient and clinician to some Hg vapor during or post-procedure.
Always polish amalgams while using water to lubricate and cool the surface. Also, utilize high-volume evacuation to ensure that any mercury-rich materials that form vapor are quickly eliminated and not inhaled by the patient or the dental hygienist.
Amalgam restorations do wear, albeit very slowly. They also expand slowly over time. The net change on occlusal surfaces is that there is no visible change. Yet, in sites protected from intraoral food abrasion, such as interproximal surfaces or facial surfaces, amalgams may slowly begin to stand out from the cavity above the surface of adjacent tooth structure.6 This appearance is not the result of bad dentistry, but simply the lack of natural abrasion. Use water cooling when resurfacing an amalgam restoration. Resurfacing an amalgam restoration may release some Hg vapor, so local high-volume evacuation should be utilized as well as a rubber dam to remove any associated liquid or vapor.
Infiltrated interproximal lesions. Interproximal lesions without cavitation can be infused with special low-viscosity resin that is polymerizable to halt lesion progression and reinforce tooth structure. Beware that radiolucencies on intraoral radiographs may not necessarily signify an advancing carious lesion. These infiltrations stop caries.8 The process should be noted carefully in the patient record during placement. Check for those treatments in the patient’s history.
Titanium implant posts and titanium alloys. These materials are protected by a film of titanium dioxide (called a passivating film) that forms rapidly, clings tightly to the surface. It is so thin that it appears transparent and invisible. Scaling or aggressive polishing procedures remove this protective film. It will immediately reform if the surface is clean. Effective but not overly aggressive instrumentation strokes should be used, along with light polishing pressure and pumice and water. The same protective film may be disturbed by acidic reactions associated with some topical fluorides.
Ceramic: all-ceramic and porcelain-fused-to-metal restorations. Ceramic is relatively resistant to degradation but surfaces can be partly dissolved by highly acidic intraoral solutions.9–11 Treatments with certain topical fluorides will also dissolve small bits of the surface. We have found that coating at-risk surfaces with a petroleum jelly film or other nonwater soluble agent is a simple way to provide temporary protection.
As delivered, ceramic materials should have very smooth external surfaces. Any intraoral adjustments produce surface scratches that require smoothening with diamond finishing pastes (particle sizes approaching 0.1 µm). Ceramics have high hardness and therefore require zirconia or diamond polishing agents for smoothening. Ceramics are very susceptible to crack formation from stressed areas containing surface defects. If these defects are recognized, they should be removed with special small particle diamond polishing pastes. They cannot be removed with normal polishing materials during a dental hygiene procedure.
Dental cements. Permanent restorations are attached with traditional acid-based cements, glass ionomer cements, or resin (composite) cements. The thickness of exposed cement at margins is typically 50 microns to 250 microns. These materials generally have lower hardness than restorative materials or tooth structure. Aggressive polishing may force abrasive material into the margin and potentially erode the cement.12 A technique of light pressure with prophy cup and prophy paste while polishing with swiping strokes across the margins is recommended.
Denture base materials. Most denture base polymer is PMMA with a hardness value similar to that of dentin. These materials are routinely cleaned with commercial denture base products and/or a soft toothbrush with soap and water. Abrasives in polishing materials or dentifrices will produce some surface scratching. Denture base material is commonly crosslinked by adding some difunctional monomer with the original methyl methacrylate monomers, which creates a more water-resistant material. Remember that a PMMA denture is prone to absorb water intraorally, and lose water when it is out of the mouth. Its mechanical properties vary as a function of water content. This is why dentures are stored in water when not being worn. Avoid procedures that would dry out the material.
Gold alloys. Alloys based on gold also corrode, but very slowly. Their surfaces are susceptible to electrochemical corrosion and over time may develop some surface pitting due to the presence of plaque. Therefore, gold restorations should be fully polished during recare visits. Rubber cup polishing with fine prophy paste is recommended. Any surface corrosion products that form are water soluble and, therefore, will not accumulate. Areas that are pitted can be polished, but will continue to slowly corrode if not kept clean.
Bonding systems. Bonding agents are extremely thin (< 5 µm) and only exposed at margins. Bonding agents are not at risk during routine prophylaxis and polishing operations. Do not attempt to remove stain that has crept into open margins of composites or veneers because of the possibility of damaging the margins. This situation is an esthetic failure and requires repair of margins.
CONCLUSION
Dental hygienists play a crucial role in the long-term maintenance of dental restorations. Appropriate care of restorations during dental hygiene procedures depends on recognizing restorative materials, understanding specific precautions, and carefully conducting finishing/polishing procedures. Removal of stain and plaque depends on the hardness of the prophylaxis agent, which should always be less than the hardness of the phases involved in the restorative material or tooth structure. The goal is always to remove plaque, calculus, and/or stain without disturbing the structure of underlying restorative materials.
REFERENCES
- Bayne SC, Powers JM, Swift Jr EJ, Thompson JY. Biomaterials. In: Mosby’s Comprehensive Review of Dental Hygiene. 7th ed. Darby ML, ed. St. Louis: Mosby Elsevier; 2015.
- Bayne SC. Biomaterials. In: Toothwear: The ABC of Worn Dentition. F Khan, WG Young, eds. West Sussex, United Kingdom: Wiley-Blackwell; 2011:153–167.
- Bayne SC, Taylor DF, Heymann HO. Protection hypothesis for composite wear. Dent Mater. 1992;8:305–309.
- Wilder AD, Bayne SC, Perdigao J, Heymann HO, Swift EJ. 10-Year clinical performance of packable posterior composite. J Dent Res. 2008;87(Spec Iss B):0125.
- Wilder AD, May KN, Bayne SC, Taylor DF, Leinfelder KF. Seventeen-year clinical study of ultraviolet-cured posterior composite Class I and II restorations. J Esthet Dent. 1999;11:135–142.
- Bayne SC, Thompson JY. Biomaterials. In: Sturdevant’s Art and Science of Operative Dentistry. 5th ed. Roberson TM, ed. St.Louis: Mosby. 2006:135–242.
- Hansen M, Anderko K. Constitution of Binary Alloys. 2nd ed. New York: McGraw-Hill, New York; 1958.
- Peters MC, Hopkins AR Jr, Yu Q. Resin infiltration: an effective strategy for managing high caries risk—a within-person randomized controlled clinical trial. J Dent. 2018;79:24–30.
- Sposetti VJ, Shen C, Levin AC. The effect of topical fluoride application on porcelain restorations. J Prosthet Dent. 1986;55:677–682.
- Abbassy MA. Fluoride influences nickel-titanium orthodontic wires’ surface texture and friction. J Orthod Sci. 2016;l54:121–126.
- Ccahuana VZ, Ozcan M, Mesquita AM, Nishioka RS, Kimpara ET, Bottino MA. Surface degradation of glass ceramics after exposure to acidulated phosphate fluoride. J Appl Oral Sci. 2010;18:155-65.
- Kimyai S, Azar FP, Daneshpooy M, Kahnamoli MA, Davoodi F. Effect of two prophylaxis methods on marginal gap of Cl V resin-modified glass-ionomer restorations. Dent Res Dent Clin Dent Prosp. 2016;10:23–29.
From Dimensions of Dental Hygiene. January 2019;17(1):32–35.



