
Ultrasonics Unveiled
Anna M. Pattison, RDH, MS, provides a broad-based look at ultrasonic instrumentation as well as a brief discussion of new products in the ultrasonic armamentarium.
Q. Which ultrasonic tips do you prefer for heavy calculus removal?
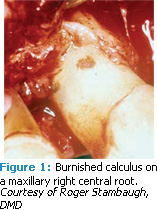
A. Ultrasonic tip selection depends on the type and the tenacity of the calculus you are trying to remove. Heavy supragingival calculus or heavy subgingival calculus that is not highly calcified is less tenacious than highly calcified older rings or ledges of subgingival calculus, which is more difficult to remove.1 Very hard, tenacious, sheet-like calculus is the most difficult to remove because it has been burnished by previous ultrasonic or hand instrumentation and smoothed over into a lump, bump, or sheet—making it more difficult to remove than when the calculus was in its original untouched state (Figure 1).
For these latter types of more tenacious calculus, four steps are necessary for successful, efficient removal:
- Adequate tip size/metal mass,
- Beveled tip geometry/shape,
- High power, and
- A tapping technique.
Tip Size/Mass
A significant amount of metal mass must be present in the tip to transmit enough strong ultrasonic vibration to break through or fracture the calculus. The tip should be large, able to withstand high power (if it’s a thinner tip), or powerful enough to fracture thick or tenacious calculus (Figure 2).
Tip Geometry/Shape
Whether you are using a magnetostrictive or a piezoelectric tip, the tip geometry in cross section is critical for effective removal of tenacious calculus. The tip should have beveled edges or a straight edge with a corner. Any beveled 1000 magnetostrictive tip usually performs better than a smooth 10 tip that is round in cross section (Figures 3A-3E).
A flat, triangular “#1,” “A,” or “P” type piezoelectric tip that has an angular edge (Figures 4A-4B) will engage and remove this type of calculus better than a large piezo tip that is round in cross section.
Power Level
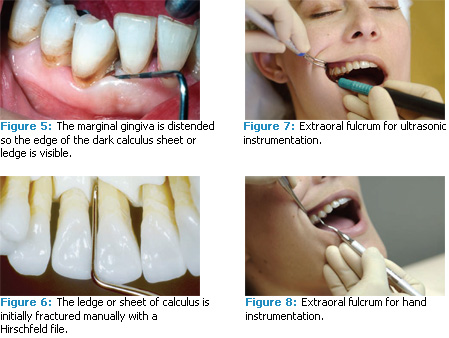
High enough power must be used to break the calculus. Make sure your unit can be turned up to high power—keep turning it up in increments until you know that it can break through the hard, tenacious calculus. Find an area where the tissue is loose enough so you can distend the marginal gingiva and see the edge of the dark calculus sheet or ledge (Figure 5). Determine whether the tip is actually cracking the calculus away from the root surface. Since you can usually only see down a few millimeters subgingivally, keep checking with the point of an explorer in an up and down vertical stroke to make sure that the calculus is breaking cleanly away from the tooth and not just being burnished into a thin sheet or a smooth lump.
Tapping Technique
Use a tapping technique to fracture the coronal edge of the deposit. Keep the ultrasonic tip parallel with the root surface and use the point as if it were a periodontal probe activated with a vertical probing stroke. Start at the coronal border of the calculus and work your way down the root. Systematically break away the calculus in a horizontal row a few millimeters down from the coronal edge. Move across a section of the root horizontally and then go a little deeper and repeat the process so the deposit is removed in a series of horizontal channels. Be careful at the base of the pocket because you can penetrate through the soft tissue attachment and expose the crest of bone.
Another highly effective technique is to initially fracture the ledge or sheet of calculus manually with a periodontal file and subsequently attack it with an ultrasonic tip on medium high to high power (Figure 6). By cracking the surface of the calculus first, the adherence to the root surface is weakened, requiring less effort to completely remove the deposit when followed by ultrasonic scaling or additional hand instrumentation.
The Use of Extraoral Fulcrums
Q. Many dental hygienists love the ultrasonic scaler because they can use extraoral fulcrums to access maxillary pockets much easier than hand instruments with intraoral fulcrums. Can extraoral fulcrums be used with hand instruments?
A. Extraoral fulcrums can be used safely with both hand instruments and ultrasonic scalers. I have been teaching extraoral and alternative fulcrums for hand instrumentation to both dental hygiene and dental students for more than 30 years at the USC School of Dentistry with no problems.
Extraoral fulcrums are commonly used to obtain access and proper angulation in deep pockets. Limiting yourself to the exclusive use of intraoral fulcrums is crippling because it usually results in short, superficial scaling strokes that only extend 3 mm to 4 mm subgingivally. In order to scale deeper with a close intraoral rest, you have to begin finger flexing, which is ergonomically unsound. If you try to extend deeper with a wrist rocking stroke, the blade angulation will close at the completion of each stroke—burnishing the calculus.
 The position of your grasp on the handle, your body position, and the placement of your hand on the patient’s face are the keys to effectively using extraoral fulcrums in both ultrasonic and hand scaling.2 The same body positions and fulcrums you use with the ultrasonic scaler for maxillary teeth will work very successfully with hand instruments (Figures 7 and 8). If there is tenacious calculus on the tooth, you may need to reinforce your extraoral fulcrum during hand instrumentation with the index finger of your other hand (Figure 9).
The position of your grasp on the handle, your body position, and the placement of your hand on the patient’s face are the keys to effectively using extraoral fulcrums in both ultrasonic and hand scaling.2 The same body positions and fulcrums you use with the ultrasonic scaler for maxillary teeth will work very successfully with hand instruments (Figures 7 and 8). If there is tenacious calculus on the tooth, you may need to reinforce your extraoral fulcrum during hand instrumentation with the index finger of your other hand (Figure 9).
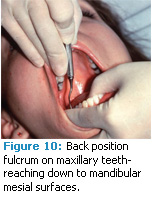
On the mandibular arch, you can use either type of fulcrum with ultrasonic scaling. Hand scaling offers many alternative fulcrums such as moving to a back position and establishing your fulcrum on the maxillary teeth (Figure 10).
Burnished Calculus
Q. How can you tell if you have thoroughly removed the calculus or just burnished it with the ultrasonic tip if you don’t have an endoscope to see subgingivally?
A. Without an endoscope it is very difficult to know definitively because we can only use tactile assessment with a sharp explorer or curet.
Years ago when I was a student at USC, the best clinical instructors would feel with a sharp explorer and insist on glassy, smooth root surfaces. We now know through endoscopy that they were feeling very clean root surfaces and, most likely, all the embedded residual calculus was gone. At that time it was the best immediate, empirical evidence they had without laying a surgical flap. Today, we can examine subgingivally with the endoscope to see if anything is left. Since less than 300 endoscopes are currently in use, your best indication of success is lack of bleeding on probing.3,4 Although this is not 100% foolproof, if there is still bleeding on probing when you reevaluate after 4 weeks the treatment team should decide if a referral to a periodontist is the next step. If the patient is not referred, all of the bleeding areas need to be rescaled.
Probe and explore the bleeding pockets carefully by going up and down vertically with the point of an explorer. Burnished calculus will feel different with a leather-like texture rather than a clean, hard root surface. Often you can feel the coronal edge of the burnished calculus sheet with the point of the explorer on the down stroke in the apical direction but not on the up stroke in the coronal direction. Use anesthesia if necessary and pay particular attention to the base of the pocket, developmental depressions, roughness at the CEJ, and depressions adjacent to furcations. These are areas that typically have residual burnished calculus when examined with the endoscope.5-7 Reappoint the patient for another reevaluation after 4 weeks or examine again at the next 3-month maintenance appointment. If the pockets are still bleeding after one or two attempts at rescaling, explain the importance of eliminating chronic periodontal infection and inflammation and refer the patient to a periodontist.8-13
Q.Why do the burnished pieces of calculus need to be removed if cavitation kills the bacteria in the pocket? Aren’t the acoustic streaming and lavage from ultrasonic scaling enough to insure a good clinical result even if calculus is left behind?
A. This depends entirely on how much calculus is left behind. Although it is impossible to remove 100% of the calculus from the tooth, all clinicians should strive to remove as much calculus as possible. Classic periodontal studies show that as much as 50% of subgingival calculus is left after initial deep scaling.14-17 Scaling with the aid of a dental endoscope can result in removal of 99% of the calculus, with only 1% of residual burnished calculus left primarily at the CEJ.18 Whenever even small, smooth pieces of calculus remain, bacteria embedded in the porous surface of the calculus soon begin proliferating and recolonizing. Within hours, biofilm is reestablishing on the root surface.19,20
In other words, the pocket can never be entirely sterilized—not with ultrasonic scaling, hand instrumentation, irrigation, a laser, locally delivered antibiotics, periodontal surgery, or even systemic antibiotics. All of these therapies can temporarily and even profoundly decrease the number of bacteria and pathogens in the pocket but none can ever eradicate them completely.21-32 Biofilm inevitably recolonizes the subgingival root surfaces within days and pathogens can return to levels that will incite inflammation within a few weeks.33,34
The idea that ultrasonic cavitation kills pathogens in the periodontal pocket is a widely held myth that has no scientific proof. This is based solely on laboratory (in vitro) experiments that are usually conducted in beakers filled with water and planktonic bacteria.35-38 Baehni and coworkers found a marked decrease in pathogens after ultrasonic scaling but this effect was most likely due to skillful mechanical debridement and lavage rather than cavitation actually killing the pathogens.35 Cavitation has never been shown to occur in the periodontal pocket space because dental ultrasonic tips are not capable of generating the intense cavitation required to kill the various pathogens.36
One study showed that a very powerful laboratory ultrasonic cell disruptor with a huge tip the size of a human finger was able to produce cavitation that killed pathogens in water in a beaker after 2.5 minutes. The ultrasonic scaler used in this study did not kill any of the tested periodontal pathogens. There are no dental ultrasonic scalers powerful enough to produce this kind of cavitational effect within the periodontal pocket.36 When bacteria were killed in another laboratory study, researchers again concluded that cavitation did not occur. The bacteria may have died because the deflected water flow and minimal lavage in this experiment caused a rise in temperature around the tip.37 There must be enough power and liquid between the tip and the tooth or tissue for true cavitation to occur.37Clearly more research is necessary to understand cavitation in the pocket.
A decrease in a pocket’s bacteria levels is caused mostly by the physical disruption of calculus and biofilm plus the lavage created by the water flow (acoustic streaming).37 Ample water flow and thorough flushing of calculus and bacteria from the pocket are some of the best features of ultrasonic scaling and they are critical to its ultimate success.
Introduction of New Products
Q.What are the newest developments in magnetostrictive ultrasonic units or tips?
A. Hu-Friedy has just introduced a powerful new magnetostrictive ultrasonic unit called the SWERV3™ (Figure 11). It provides a full range of power from very low power for gentle debridement to high power for effective heavy calculus removal. There is less chance of burnishing calculus with the SWERV3 because it is engineered to deliver reliable high power to any standard, large insert to remove calculus quickly and efficiently. The new unit also offers a flat touch pad control panel to insure effective infection control. Any of the Hu-Friedy tips work with this device and it also accepts magnetostrictive inserts from other manufacturers.
DENTSPLY Cavitron’s newest insert is an ultra thin tip called the ThinSert (Figure 12). It is ideal for biofilm removal on patients who have tight tissue or close root proximity. This tip is half the diameter of a regular SlimLine tip. When a SlimLine tip is too thick to reach into a tight spot, the ThinSert slips right in. Even though this is the thinnest tip made by DENTSPLY, it is specially designed so it can be turned up to high power without danger of breaking.
 The Burnett Power Tip by Parkell is another thin tip that is manufactured for use on high power for more efficient calculus removal (Figure 2). When used in the small but powerful Parkell magnetostrictive unit, it provides enough amplitude to break through calculus.
The Burnett Power Tip by Parkell is another thin tip that is manufactured for use on high power for more efficient calculus removal (Figure 2). When used in the small but powerful Parkell magnetostrictive unit, it provides enough amplitude to break through calculus.
Brasseler USA has just introduced a new line of 16 magnetostrictive inserts called Hygiene- Pro (Figure 13). They offer ergonomic grip designs in both hard and soft styles with nonslip surfaces to reduce hand fatigue.
Q.What are the newest developments in piezoelectric ultrasonic units or tips?</h3
A. PDT (Paradise Dental Technologies) recently introduced a new type of piezoelectric tip from France called the Scorpion. The Scorpion insert tips are coated with a 2µm thick layer of gold-colored titanium nitride that serves as a visual indicator of wear. Once the titanium coating has disappeared, the clinician knows that the insert needs to be replaced. The Scorpion tips are very thin and designed to easily access interproximal spaces. They come in a variety of designs including the P, PS, and many other choices (Figure 14). They are available with either Satelec or EMS type threads and will work with all of the different brands of piezoelectric units.
Vista Dental has introduced a new line of Gracey Mini Curette Tips that features a long shank and slender design to improve accessibility into deeper pockets (Figure 15). The tips offer a shorter blade to allow for more efficacious scaling in narrow pockets, to limit tissue damage, and improve adaptation to the root surface. The Gracey Mini Curettes are available in three different sets of tips for use on anterior teeth, premolars, and molars.
Another innovation is the introduction of After Five piezo tips from Hu-Friedy that are designed for deeper pocket scaling. Like the After Five Gracey curets, the longer length of these tips enhance access (Figure 16).
Q. What do you think of the new, lighted ultrasonic inserts and handpieces?
A. I think the advantages of the lighted handpieces and tips are definitely worth the additional cost. The fiberoptic or LED (light emitting diode) light that is added to ultrasonic scaler handpieces and inserts is especially helpful when scaling posterior teeth or lingual surfaces. Some of the lights are strong enough to transilluminate through the gingiva so the subgingival dark calculus is visible if it appears near the margin.
The newest lighted magnetostrictive tip is the Discus Dental Insight tip, which is a swivel tip with a bright LED light in the handle. (Figure 17). This tip comes in many different tip designs and has a narrower diameter than lighted piezo handpieces or other leading magnetostrictive lighting options. DENTSPLY Professional offers the Cavitron Steri-Mate® Light, an autoclavable separate LED light sleeve with an adjustable, flexible light tube that slips over its magnetostrictive handpiece (Figure 18).
Both the Hu-Friedy Symmetry Piezo unit (Figure 19) and the Brasseler NSK unit (Figure 20) come with handpieces that have an intense, built-in, fiberoptic ring light where the tip screws into the handle. SATELEC/Acteon Group makes autoclavable, integrated LED lighted handpieces for its SATELEC Newtron piezoelectric scalers (Figure 21). The Platinum Pro-Select, made by Zila, a division of TOLMAR, also has an LED light, which is not integrated into the handpiece but rather attaches to the side. (Figure 22).
REFERENCES
- Canis MF, Kramer GM, Pameijer CM. Calculus attachment: review of the literature and ?ndings. J Periodontol. 1979;50:406.
- Pattison AM, Matsuda S, Pattison GL. Extraoral fulcrums: the essentials of using extraoral fulcrums for periodontal instrumentation. Dimensions of Dental Hygiene. October 2004;2(10):20, 21-23.
- Lang NP, Adler R, Joss A, Nyman S. Absence of bleeding on probing. An indicator of periodontal stability. J Clin Periodontol. November 1990;17(10):714-21.
- Wilson TG, Harrel SK, Nunn ME, Francis B, Webb K. The relationship between the presence of tooth-borne subgingival deposits and inflammation found with a dental endoscope. J Periodontol. 2008 Nov;79(11):2029-35.
- Stambaugh RV, Myers G, Ebling W, Beckman B, Stambaugh KA. Endoscope visualization of the subgingival dental sulcus and tooth root surface. J Periodontol. 2002;73:374-382.
- Stambaugh RV. A clinician’s three year experience with perioscopy. Compendium of Continuing Education in Dentistry. 2002;23:1061-1070.
- Pattison A, Pattison G. Periodontal instrumentation transformed. Dimensions of Dental Hygiene. 2003;1(2):18-20, 22.
- Lowenguth RA, Greenstein G. Clinical and microbiological response to nonsurgical mechanical periodontal therapy. Periodontol 2000. 1995;9:14.
- Claffey N. Decision making in periodontal therapy: the reevaluation, J Clin Periodontol. 1991;18:364.
- Joss A, Adler R, Lang NP. Bleeding on probing. A parameter for monitoring periodontal conditions in clinical practice. J Clin Periodontol. 1994 Jul;21(6):402-8.
- Lang NP, Joss A, Orsanic T, Gusberti FA, Siegrist BE. Bleeding on probing. A predictor for the progression of periodontal disease? J Clin Periodontol. 1986 Jul;13(6):590-6.
- Greenstein G. Nonsurgical periodontal therapy in 2000: a literature review, J Am Dent Assoc. 2000;131:1580.
- Cobb CM. Microbes, inflammation, scaling and root planing, and the periodontal condition. J Dent Hyg. October 2008;82(suppl 3):4-9
- Sherman PR, Hutchens LH, Jewson LG, Moriarty JM, Greco GW, McFall WT Jr. The effectiveness of subgingival scaling and root planing. I. Clinical detection of residual calculus. J Periodontol. 1990;61:3-8.
- Kepic TJ, O’Leary TJ, Kafrawy AH. Total calculus removal: an attainable objective? J Periodontol. 1990;61:16-20.
- Fleischer HC, Mellonig JT, Brayer WK, Gray JL, Barnett JD. Scaling and root planing efficacy in multirooted teeth. J Periodontol. 1989;60:402-409.
- Brayer WK, Mellonig JT, Dunlap RM, Marinak KW, Carson RE. Scaling and root planing effectiveness: the effect of root surface access and operator experience. J Periodontol. 1989:60:67-72.
- Stambaugh RV, Myers GC, Watenabe J, Lass C, Stambaugh KA. Clinical response to scaling and root planing aided by the dental endoscope [abstract]. J Dent Res. 2000;79(special issue):2762.
- Tan B, Gillam DG, Mordan NJ, Galgut PN: A preliminary investigation into the ultrastructure of dental calculus and associated bacteria. J Clin Periodontol 2004;31:364.
- Haffajee AD, Patel M, Socransky SS. Microbiological changes associated with four different periodontal therapies for the treatment of chronic periodontitis. Oral Microbiol Immunol. 2008 Apr;23(2):148-57.
- Haffajee AD, Teles RP, Socransky SS. The effect of periodontal therapy on the composition of the subgingival microbiota. Periodontol 2000. 2006;42:219-58.
- Cobb CM. Clinical significance of non-surgical periodontal therapy: an evidence-based perspective of scaling and root planing. J Clin Periodontol. 2002 May;29(suppl 2):6-16.
- Petersilka GJ, Ehmke B, Flemmig TF. Antimicrobial effects of mechanical debridement. Periodontol 2000. 2002;28:56-71.
- Haffajee AD, Cugini MA, Dibart S, Smith C, Kent RL Jr, Socransky SS. The effect of SRP on the clinical and microbiological parameters of periodontal diseases. J Clin Periodontol. 1997;24(5):324-34.
- Shiloah J, Patters MR. Repopulation of periodontal pockets by microbial pathogens in the absence of supportive therapy. J Periodontol. 1996;67(2):130-9.
- Sbordone L, Ramaglia L, Gulletta E, Iacono V. Recolonization of the subgingival microflora after scaling and root planing in human periodontitis. J Periodontol. 1990;61(9):579-84.
- Renvert S, Wikström M, Dahlén G, et al: Effect of root debridement on the elimination of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis from periodontal pockets. J Clin Periodontol. 1990;17:345.
- Garnick JJ, Dent J: A scanning electron micrographical study of root surfaces and subgingival bacteria after hand scaling and ultrasonic instrumentation. J Periodontol. 1989;60:441.
- van Winkelhoff AJ, van der Velden U, de Graaff J: Microbial succession in recolonizing deep periodontal pockets after a single course of supra-and subgingival debridement. J Clin Periodontol. 1988;15:116.
- Lavanchy DL, Bickel M, Baehni PC. The effect of plaque control after scaling and root planing on the subgingival microflora in human periodontitis. J Clin Periodontol. 1987;14(5):295-9.
- Magnusson I, Lindhe J, Yoneyama T, Liljenberg B. Recolonization of a subgingival microbiota following scaling in deep pockets. J Clin Periodontol. 1984;11(3):193-207.
- Rosenberg ES, Evian CI, Listgarten M: The composition of the subgingival microbiota after periodontal therapy. J Periodontol. 1981;52:435.
- Slots J, Mashimo P, Levine MJ, Genco RJ. Periodontal therapy in humans. I.Microbiological and clinical effects of a single course of periodontal scaling and root planing, and of adjunctive tetracycline therapy. J Periodontol. 1979;50(10):495-509.
- Wilson TG, Carnio J, Schenk R, Myers G. Absence of histologic signs of chronic inflammation following closed subgingival scaling and root planing using the dental endoscope: human biopsies – a pilot study. J Periodontol. 2008;79(11):2036-41.
- Baehni P, Thilo B, Chapuis B, Pernet D. Effects of ultrasonic and sonic scalers on dental plaque microflora in vitro and in vivo. J Clin Periodontol. 1992;19(7):455-9.
- Schenk G, Flemmig TF, Lob S, Ruckdeschel G, Hickel R. Lack of antimicrobial effect on periodontopathic bacteria by ultrasonic and sonic scalers in vitro. J Clin Periodontol. 2000;27(2):116-9.
- O’Leary R, Sved AM, Davies EH, Leighton TG, Wilson M, Kieser JB. The bactericidal effects of dental ultrasound on Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. An in vitro investigation. J Clin Periodontol. 1997;24(6):432-9.
- Trenter SC, Landini G, Walmsley AD. Effect of loading on the vibration characteristics of thin magnetostrictive ultrasonic scaler inserts. J Periodontol. 2003;74(9):1308-15.
From Dimensions of Dental Hygiene. April 2010; 8(4): 36-45.

