
The Importance of Complete Root Coverage
When using ultrasonic instrumentation, the entire root surface must be meticulously debrided in order to achieve successful outcomes.

FIGURE 1B. An ultrasonic insert/tip is activated on medium power and positioned apically, similar to a probe.
Ultrasonic technology has a prominent place in a nonsurgical periodontal therapy armamentarium with good reason.1,2 Ultrasonic therapy is easy to use and requires less physical effort than hand instrumentation.3 Power scaling provides clinicians with ergonomic benefits by reducing the amount of cumulative stress typically experienced during hand instrumentation.4,5 Another advantage is the ability of the ultrasonic insert/tip (UIT) to shatter calculus with a simple touch when used properly at the correct setting.6 These benefits often bolster the misconception that power instrumentation is fast and easy. Ultrasonic therapy does not provide a shortcut in the delivery of clinical care, as meticulous coverage of the root surface is necessary to achieve definitive periodontal debridement.
A haphazard and hasty approach, often caused by time limitations or clinician inexperience, is likely to result in the partial scaling or burnishing of subgingival calculus.7 Leaving behind any amount of biofilm and/or mineralized deposit on the root surface can cause sustained inflammation in the overlying tissue, as demonstrated through endoscopy.8–15
SEEING IS BELIEVING
One of the most valuable learning tools for the use of ultrasonic technology is a simple coverage exercise. This exercise uses an extracted tooth (without amalgam16,17) that has been autoclaved18,19 and painted with black nail polish or hobby paint 360° at the cervix to simulate 6 mm or more of attachment loss (Figure 1).
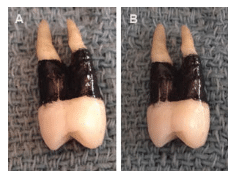
FIGURE 2B. A second vertical stroke delineates the extent of the channel for each debridement segment.
Activate the UIT directed apically on the extracted tooth’s painted surface and perform one stroke (Figure 2). Note the track of debridement that has been achieved and where the energy is confined at the terminal millimeters of the tip (generally 2 mm or less due to the contrasting surface characteristics of the UIT’s straighter edge and the tooth’s curvature).20
Practice adapting the tip of the UIT to various contours of the tooth to see the stroke achieved in a concavity, furcation, and line angle. Remember the goal is to achieve comprehensive surface coverage, with specks of paint mimicking the biofilm and/or calculus left behind. Practice two different approaches: random scribbles vs overlapping vertical channels, noting the relative efficiency of each method (Figure 3).
Now repeat the exercise on a freshly painted surface, but this time position the tip as though the gingival tissues were intact to the cementoenamel junction. Observe the number of strokes needed to achieve complete removal of the paint. This portion of the exercise demonstrates the limits of adaptation in more severe root curvatures, such as deep concavities and/or furcations.6 These limitations support the need for follow-up with small curvature-bladed hand instruments that can adapt to these difficult-to-reach contours.21
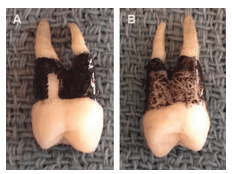
FIGURE 3B. The random scribble method leaves significant root contaminants behind, limiting healing in the overlying soft tissue.
DIAGRAM APPROACH
Another exercise that illustrates the time necessary to achieve comprehensive coverage can be done with a diagram. Keep in mind, however, that the flat surface of paper in no way mimics the organic contours of tooth roots, so much more time is needed to achieve these results in vivo.
Figure 4 depicts the subgingival space of two teeth with varying levels of disease. Using a dull pencil that simulates the stroke width obtained on the painted tooth exercise, fill in the space between the lines to completely blacken the area. Note the speed of movement used in both exercises. Although biofilm can be rapidly dispersed with brief contact, dense calculus is removed more effectively with slower UIT movement and prolonged contact to maximize the energy transfer. Therefore, in a well-maintained patient with no bleeding on probing, the task is biofilm removal, which can be accomplished with rapid strokes—as long as there is comprehensive coverage of the root surface. When calculus is present, a moderate to slow pace of UIT movement will reduce the likelihood of burnishing deposits and deliver a more thorough result.
CONCLUSION
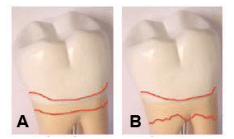
FIGURE 4B. This image depicts more severe periodontal involvement.
Complete root overage cannot be underestimated as a key principle of ultrasonic instrumentation, as incomplete coverage is often to blame for poor clinical outcomes. The good news is that simple awareness coupled with self-evaluation easily remedy this error. Clinicians who apply the insights gained from these exercises will ensure that the farthest reaches of subgingival space are adequately debrided using ultrasonic and hand instrumentation. Concentrating on technique and coverage using methodical overlapping strokes to reach every square millimeter of root surface will produce optimal results for clinicians and patients.
REFERENCES
- Research, Science, and Therapy Committee of the American Academy of Periodontology. Position paper: sonic and ultrasonic scalers in periodontics. J Periodontol. 2000;71:1792–1801.
- Arabaci T, Cicek Y, Canackci CF. Sonic and ultrasonic scalers in periodontal treatment: a review. Int J Dent Hyg. 2007;5:2–12.
- Murphy D. Ergonomics and the Dental Care Worker. Washington, DC: American Public Health Association; 1998:61,432,446.
- Bramson JB, Smith S, Romagnoli G. Evaluating dental office ergonomic risk factors and hazards. J Am Dent Assoc. 1998;129:174–183.
- Zakaria D, Robertson J. Work-related cumulative trauma disorders of the upper extremity. Am J Ind Med. 2002;42:258–269.
- Matsuda SA. A practical look at ultrasonic instrumentation. Dimensions of Dental Hygiene. 2009;7(7):32–34.
- Brayer WK, Mellonig JT, Dunlap RM, Marinak KW, Carson RE. Scaling and root planing effectiveness: the effect of root surface access and operator experience. J Periodontol. 1989:60:67–72.
- Sbordone L, Ramaglia L, Guletta E, Iacono V. Recolonization of the subgingival microflora after scaling and root planing in human periodontitis. J Periodontol. 1990;61:579–584.
- Fujikawa K, O’Leary TJ, Kafrawy AH. The effect of retained subgingival calculus on healing after flap surgery. J Periodontol. 1988;59:170–175.
- Checchi L, Montevecchi M, Checchi V, Zappulla F. The relationship between bleeding on probing and subgingival deposits. An endoscopical evaluation. Open Dent J. 2009;28:154–160.
- Stambaugh RV, Myers GC, Watenabe J, Lass C, Stambaugh KA. Clinical response to scaling and root planing aided by the dental endoscope. J Dent Res. 2000;79:2762.
- Pattison A, Pattison G. Periodontal instrumentation transformed. Dimensions of Dental Hygiene. 2003;1(2):18–22.
- Chen C. Periodontitis as a biofilm infection. J Calif Dent Assoc. 2001;29:362–369.
- Pattison AM. Ultrasonics unveiled. Dimensions of Dental Hygiene. 2010;8(4):36–45.
- Pattison AM. Keys to effective calculus removal. Dimensions of Dental Hygiene. 2011;9(10):50–53.
- Parsell DE, Karns L, Buchanan WT, Johnson RB. Mercury release during autoclave sterilization of amalgam. J Dent Educ.1996;60:453–458.
- Kumar M, Sequeira PS, Peter S, Bhat GK. Sterilisation of extracted human teeth for educational use. Indian J Med Microbiol. 2005;23:256–258.
- Tate WH, White RR. Disinfection of human teeth for educational purposes. J Dent Educ. 1991;55:583–585.
- Pantera EA Jr, Schuster GS. Sterilization of extracted human teeth. J Dent Educ. 1990;54:283–285.
- Pattison AM. The right stuff. Dimensions of Dental Hygiene. 2015;13(1):31–37.
- Matsuda SA. The pathway to synergy. Dimensions of Dental Hygiene. 2008;6(7):24–26.
From Dimensions of Dental Hygiene. October 2015;13(10):36,38–39.

