
The Hidden Threat
Enamel erosion is caused by lifestyle habits common among most patients. Effective risk assessment and patient education are key to preventing serious and irreversible damage.
This course was published in the December 2010 issue and expires December 2013. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- List the extrinsic factors impacting erosion.
- Describe the significance of pH levels and titratable acidity.
- Identify various methods to decrease the risk of erosion.
- Evaluate acid exposure when conducting risk assessments.
One of the practicing dental hygienist’s most important responsibilities is performing a caries and periodontal disease risk assessment, which includes taking the patient’s health history and conducting a clinical evaluation. Enamel erosion should also be part of every patient’s risk assessment.1Many patients are unaware of the danger inherent in many of their daily habits. It is up to dental hygienists to make patients aware of the damage that can occur and advise them on prevention strategies.
Erosion is not a new phenomenon. Research has long shown that regurgitation, such as vomiting and/or gastroesophageal reflux disease, can cause tooth erosion.2 These intrinsic factors can cause significant damage to the tooth structure and can be addressed through medical intervention. Systemic conditions and salivary flow and content may also play a role in erosion. However, dental erosion is also a serious threat to many people who may not even be aware of their risk. Mundane activities such as consuming acidic foods, beverages, and medications; swimming in chlorinated pools; and undergoing tooth whitening procedures can all cause enamel erosion.2,3 Dental hygienists can help their patients reduce the risk through effective patient education and strategies to mitigate these behaviors’ effects on tooth enamel.
EPIDEMIOLOGY, RISK FACTORS, AND IMPACT
Dental erosion is caused by the weakening of the outer layer of mineralized tissue, which increases the risk of tooth wear by intrinsic and extrinsic acids. This weakening results in the removal of tooth structure that cannot be reversed. 4 Erosion is one of the leading causes of demineralization and dentinal hypersensitivity. Educating patients about its causes is crucial because often erosion is not recognized until the irreversible damage is complete.2A neutral pH level in the mouth can range from 6.75 to 7.25.5 Tooth structure begins to soften and demineralize when the pH levels fall between 6.0 and 5.5.6
The lower the pH level, the higher the acidity level, thus, when people consume foods and beverages with high acidity, their risk of erosion increases. Patients who have recession are also at greater risk of erosion because cementum is softer in composition. However, the inclusion of calcium, fluoride, or phosphorous at the time of the acid exposure may reduce the risk of erosion, regardless of the pH level, due to the remineralization properties of these minerals.2,7-9

The frequency of acid exposure is important because the more exposure, the higher the risk of erosion. 2,8 The method of ingestion may also be noteworthy; some researchers suggest drinking through a straw when consuming acidic beverages may decrease the risk of erosion because the dentition is not bathed in the beverage.2 Titratable acidity (TA), which is the time needed for the decreased pH level to return to the resting level, is also a factor in erosion.8
THINK BEFORE YOU DRINK
The exposure of dentition to acidic beverages is a significant cause of dental erosion, and the consumption of acidic beverages continues to rise. In the United States from 1977 to 1997, soda consumption increased 61%, fruit juice consumption increased 42%, and wine consumption increased 11%.10
Many patients only consider soda’s correlation to tooth decay, but the acidity of soda may also cause serious harm to the dentition. Sports drinks, energy drinks, flavored waters, and wine are also contributors to erosion.7,8,9,11,12
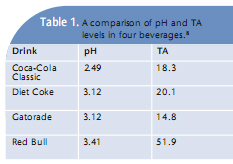
Other beverages that may cause acid wear include smoothies and some flavored coffees and teas. Both the pH and TA levels are key factors in determining erosivity. One study analyzed the pH and TA of four beverages and then compared the extent of erosion. The results are listed in Table 1. Each of these beverages created enamel erosion to some extent. The starting pH of Red Bull Energy Drink was the highest out of the four, meaning it was the least acidic, but it had the highest TA level and created the most erosion damage. 8 This study illustrates that the pH level is not the only cause of erosion; additional factors must be considered. Another study examined enamel erosion using a sports drink containing calcium, and compared it to a commercial sports drink and water. The calcium-containing sports drink was developed to reduce the amount of erosion caused by sports drinks.9 The results demonstrated great promise. The pH of the water was 6.79, the pH of the calcium-containing drink was 3.81, and the commercial sports drink pH was 3.16. The results confirmed no difference in enamel loss between the calcium-containing drink and the water, however, the commercial sports drink caused 30 times more erosion compared to the water and calcium-containing drink.9 This study demonstrates that the addition of calcium mitigates the acidic impact of the sports drink, so much so that the results are nearly identical to those of water. On the reverse side, when calcium was not present in the traditional sports drink, the expected outcome—erosion damage—occurred.9

All alcoholic beverages can cause erosion if consumed frequently enough, but one of the greatest concerns is wine consumption. 11,13 The pH levels of wine range from 2.3 to 3.8; beer pH levels range from 4.0 to 5.0.13 When an acidic beverage is consumed with food, the risk of erosion is decreased because some foods, such as proteins or fats, offer a neutralizing mechanism to the tooth surface and stimulate salivary flow.
The risk of erosion increases when an acidic beverage is consumed without food and over time. This puts leisurely wine drinkers and professional wine tasters at high risk. Wine tasters may sample wines several times a day and hold the wine in their mouths for up to a minute at a time making their risk of erosion significant.11
Water is the best resource for quenching thirst, especially from a dental standpoint, but it is often not the preferred beverage. Given the flavorless taste of water and the attraction to bottled water, there are several alternatives available including a wide range of flavored waters. Although flavored water may appeal to the taste buds, some products pose the same risks.12 Reading the label of flavored water is essential for protecting teeth.
Many of the flavored waters contain citric acid or some other form of acid to create a pleasant and desirable taste.12 The pH levels of many flavored waters range from 2.74 to 3.34.8,12 The perception among consumers that flavored water is as harmless to teeth as unflavored water is false. It is up to dental professionals to inform consumers of the risks associated with their consumption.
ADDITIONAL EXTRINSIC FACTORS

Erosive extrinsic factors also include food, medication, tooth whitening agents, and environmental exposure. Table 2 lists the pH levels of a variety of sources of erosion. Sour candies, sour gums, and sour mints have a pH range between 2.3 and 3.14, which is more acidic than orange juice (pH 3.6).14 Saliva is also not able to act as a buffer and help restore pH levels to the resting range as easily after the consumption of sour candies compared to traditional candy flavors.15 Fruit is part of a healthy diet but it can also create erosion. The pH levels of fruit range from 2.8 to 5.2.13 Even vegetables can create erosion with pH levels between 3.9 and 5.1.13
Various medications can create a highly acidic environment as well, such as chewable vitamin C tablets and aspirin.11 One analysis of various pediatric medications found that many contained large amounts of sugar in addition to low pH levels and high TA levels.16 Whitening agents are also culprits in dental erosion. An analysis of 35 whitening agents revealed pH levels ranging from 3.67 to 11.13.3 Although some agents were deemed safe, others were highly acidic and damaging. An in vitro study concluded that tooth whitening agents re sult in some reduction of tooth structure, creating a rougher surface, with the 35% hydrogen peroxide whitening agents creating the most damage.17
Another study assessed the use of fluoride within whitening agents compared to nonfluoridated whitening agents. Results demonstrated a reduction in harm to enamel structure when fluoride was part of the whitening product.18
Although there are mixed results among studies examining the impact of whitening agents on erosion, there is sufficient evidence to warrant consideration of pH levels as well as whether or not fluoride is present in whitening products when performing an erosion risk assessment. Battery manufacturers and galvanizing workers are at risk of erosion due to their daily exposure to acid.19 Another risk is swimming.19 A healthy and balanced pool has an average water pH of 7.5. If the water is over-chlorinated, the pH level could be less than 3.0.20 Given that most swimmers take in mouthfuls of water during the duration of their time in the pool, the water pH level in swimming pools could contribute to erosion of tooth structure.
PUTTING IT INTO PRACTICE

Extrinsic factors alone put the average person at risk of erosion due to the acidity in foods and beverages. For those who take medications, experience xerostomia, and are medically compromised, their risk of erosion is increased. As a dental hygienist, it is paramount to ask patients questions about their daily routines. Find out if they are drinking sports drinks each afternoon at the gym or snacking on fruit several times a day. Asking appropriate questions about habits is a great way to investigate each patient’s risk.
Educating all patients, regardless of their risk, will help create awareness. Patients should also be advised not to brush for at least 30 minutes after an acid exposure. This includes drinking acidic beverages, eating acidic fruit, vomiting, swimming, etc. Brushing after an acid exposure significantly increases erosion because the acid is brushed into the tooth structure. Instead, swishing with water will assist in removing the taste and increase the pH level present in the mouth. Dental hygienists can then use a patient’s risk assessment to determine the most appropriate prophy paste to use and whether selective teeth should be polished or the entire dentition. Coarse polishing paste will remove more enamel than fine polishing paste. If a patient is at a high erosion risk, it may be best to not polish at all. Another possibility is the use of a polishing paste that contains fluoride, calcium, and phosphate, which can help remineralize the enamel surface.21
Furthermore, fluoride treatments are a great recommendation for patients with frequent exposures to an acidic environment. Table 3 provides a list of patient recommendations. Performing thorough medical histories that include lifestyle habits can help dental hygienists understand each patient’s level of risk relative to dental erosion. With effective patient education about the significant risks posed by some drinks, foods, and behaviors, in addition to clinical intervention such as fluoride treatment and calcium phosphate technologies, dental hygienists can help their patients maintain healthy enamel.
REFERENCES
- American Dental Hygienists’ Association. Standards for clinical dental hygiene practice. Available at: www.adha.org/downloads/adha_standards08.pdf. Accessed November 19, 2010.
- Gandara BK, Truelove EL. Diagnosis and management of dental erosion. J Contemp Dent Pract. 1999;1:16-23.
- Price RBT, Sedarous M, Hiltz G. The pH of tooth-whitening products. J Can Dent Assoc. 2000;66:421-426.
- Bahmise CT, Olusile AO, Oginni AO. An analysis of the etiological and predisposing factors related to dentin hypersensitivity. J Contemp Dent Pract. 2008;9:52-59.
- Fejerskov O, Kidd E. Dental Caries: The Disease and its Clinical Management. 2nd ed. Ames, Iowa: Blackwell Munksgaard; 2008.
- Stookey GK. The effect of saliva on dental caries. J Am Dent Assoc. 2008;139:11S-17S.
- Jager DH, Vieira AM, Ruban JL, Huysmans MC. Influence of beverage composition on the results of erosive potential measurement by different measurement techniques. Caries Res. 2008;42:98-104.
- Owens BM, Kitchens M. The erosive potential of soft drinks on enamel surface substrate: An in vitro scanning electron microscopy investigation. J Contemp Dent Pract. 2007;8:11-20.
- Venables MC, Shaw L, Jeukendrup AE, et al. Erosive effect of a new sports drink on dental enamel during exercise. Med Sci Sports Exerc. 2005;37:39-44.
- Putnam JJ, Allshouse JE. Food consumption, prices, and expenditures, 1970-97. Available at: www.ers.usda.gov/publications/sb965/sb965.pdf. Accessed November 19, 2010.
- Mandel L. Dental erosion due to wine consumption. J Am Dent Assoc. 2005;136:71-74.
- Brown CJ, Smith G, Shaw L, Parry J, Smith AJ. The erosive potential of flavoured sparkling water drinks. Int J Paediatr Dent. 2007;17:86-91.
- Cooper B. Protecting your healthy patients. Dimensions of Dental Hygiene. 2008;6(4):38-39.
- Davies R, Hunter L, Loyn T, Rees J. Sour sweets: a new type of erosive challenge? Br Dent J. 2008;204:84-85.
- Wagoner SN, Marshall TA, Qian F, Wefel JS. In vitro enamel erosion associated with commercially available original-flavor and sour versions of candies. J Am Dent Assoc. 2009;140:906-913.
- Neves BG, Farah A, Lucas E, de Sousa VP, Maia LC. Are paediatric medicines risk factors for dental caries and dental erosion? Community Dent Health. 2010;27:46-51.
- Pinto CF, de Oliveira R, Cavalli V, Giannini M. Peroxide bleaching agent effects on enamel surface microhardness, roughness and morphology. Braz Oral Res. 2004;18:306-311.
- Chen HP, Chang CH, Liu JK, Chuang SF, Yang JY. Effect of fluoride containing bleaching agents on enamel surface properties. J Dent. 2008;36:718-725.
- Wiegand A, Attin T. Occupational dental erosion from exposure to acids—a review. Occup Med. 2007;57:169-176.
- Dawes C, Boroditsky CL. Rapid and severe tooth erosion from swimming in an improperly chlorinated pool: Case report. J Can Dent Assoc. 2008;74:359-361.
- Beebe SN. To polish or not to polish? Dimensions of Dental Hygiene. 2009;7(3):32-35.
From Dimensions of Dental Hygiene. December 2010; 8(12): 52-55.



