
The Heart and Mouth Connection
While the evidence shows that periodontitis and atherosclerotic cardiovascular diseases are linked, the reasons behind this association are still being investigated.
This course was published in the February 2015 issue and expires February 2018. The author havs no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the possible mechanisms behind the association between periodontitis and atherosclerotic cardiovascular diseases.
- Identify the clinical measures of atherosclerosis that relate to periodontitis.
- Explain the effects of periodontal treatment on arterial function.
- Detail the role of C-reactive protein in periodontitis and cardiovascular disease.
Over the past 25 years, it has become apparent that oral infections/inflammation processes are associated with systemic diseases or pathologic conditions, but clear-cut causative mechanisms have not yet been established. The most common oral infections/inflammatory processes are gingivitis, periodontitis, and periapical periodontitis. In particular, chronic periodontitis has been linked to atherosclerotic cardiovascular diseases (ACVD); rheumatoid arthritis; adverse pregnancy outcomes; diabetes; and some forms of cancer.1 This review will focus on the association between periodontitis and ACVD.
Epidemiological studies have linked periodontitis with ACVD, including atherosclerosis—a subclinical form of ACVD—and cardiovascular diseases that cause an “event” (eg, myocardial infarction [MI], cerebrovascular accident [CVA], and death). An important meta-analysis published by Humphrey et al2 looked at longitudinal studies that assessed patients’ periodontal status and noted the occurrence of ACVD events in the same population. From this meta-analysis, a relative risk of 1.4 was estimated for the presence of periodontitis in relation to ACVD events. This indicates that, on average, the risk for an ACVD event in these study populations was increased by 40% if periodontitis was present at baseline.
A similar meta-analysis was performed by Dietrich et al,3 who found that men younger than 65 with periodontitis had a relative risk of 2. In other words, the presence of periodontitis among men younger than 65 doubled the risk for ACVD events. Interestingly, Humphrey et al2 showed that the increased risk for ACVD events in those subjects with periodontitis remained approximately the same, regardless of how each subject was assessed for periodontal diseases (full-mouth clinical measurements vs short questionnaires), gender, or whether the follow-up was conducted less than 10 years later or 10 years to 15 years later.
The association between periodontitis and ACVD prompted the editors of the American Journal of Cardiology and the Journal of Periodontology in 2009 to publish an editors’ consensus that the two diseases were linked, but that the mechanisms behind this association were unknown.4 Updated reviews and a joint European-American workshop concluded in 2013 came to the same conclusion.5,6 So while the two conditions are clearly associated and the epidemiological evidence shows that periodontitis always precedes ACVD events, there is insufficient evidence on a causative role of periodontitis for ACVD.
A 2010 study7 investigated the association between frequency of toothbrushing and ACVD events and included 11,869 dentate individuals who were followed for an average of 8 years. The self-reported frequency of toothbrushing was recorded (twice per day, once per day, or never), in addition to parameters of ACVD. Of the study population, 5% developed an ACVD event. Among those who brushed their teeth twice per day, 3% developed an event, while 11% of those who rarely or never brushed their teeth experienced an ACVD event. After adjusting for common risk factors, such as age, sex, smoking, and other potential confounders including, diabetes, hypertension, and socioeconomic status, the statistical significance of the results remained. The authors reported that the relative risk for an ACVD event was 1.7 times higher in those who rarely or never brushed their teeth compared to individuals who brushed their teeth twice per day.7 Thus, the irregular performance of oral hygiene procedures is related to ACVD; however, the mechanism behind this association remains unknown.
MECHANISMS BEHIND THE LINK
The possible causative role of periodontitis in the pathophysiology of ACVD is not proven. Nevertheless, a body of research has been performed to explain the epidemiological association. A summary of this evidence was presented at a workshop on periodontitis and systemic diseases held by the European Federation of Periodontology (EFP) and American Academy of Periodontology (AAP) in 2013.6
The possible causative relationship between periodontitis and ACVD is based on the fact that the inflamed periodontal pocket epithelium forms an open wound, creating a port of entry for subgingival and oral bacteria to enter the bloodstream. A healthy and intact periodontal ligament has an estimated surface area of 31 square inches to 35 square inches. The wound surface, or periodontal inflamed surface area, among individuals with periodontitis is estimated to be as large as 2 square inches to 8 square inches. In fact, the presence of daily occurring, short-lived bacteremias has been reported in many studies. Oral bacteria have been found after toothbrushing and after eating, but also spontaneously in periodontitis patients.8 Oral and periodontal bacteria have also been found in atherosclerotic lesions and arterial biopsies.8 The hypothesis is that these microorganisms in the systemic circulation may have a variety of effects, which may play a causative role in the pathophysiology of ACVD.8
The presence of a consistent pro-inflammatory state among individuals with periodontitis is another factor currently being investigated. The most studied biomarker for the pro-inflammatory state is C-reactive protein (CRP), an acute phase protein generated in the liver in response to bacterial infection and other pro-inflammatory mediators. These biomarkers must currently be regarded as “surrogate” markers for ACVD and whether they have a causative role or just “reporter” function is currently not clear. Originally, levels of CRP greater than 2.1 mg/L may have increased the risk for an ACVD event; more recent publications show levels >3 mg/L.9,10 Therefore, this pro-inflammatory state may play a role in the link between periodontitis and ACVD.
Another explanation for the association between periodontitis and ACVD is the increased immune activity in individuals with periodontitis.8 Due to frequently occurring bacteremias and transferring of microorganisms to lymphoid organs, increased antibody levels are present. These antibodies are cross-reactive with human host cells and molecules in vasculature and heart tissues. These interactions could trigger a heightened inflammatory response in the vital blood vessels, increasing the risk for ACVD events.
Yet another explanatory mechanism could be related to a prothrombotic state in periodontitis.8 Coagulation factors are normally generated in the liver, but, in periodontitis, increased levels of these coagulation factors are present. This may result in a pro-thrombotic state, increasing the risk for ischemic events.
Inflammation in general, as well as bacteremia, can trigger dyslipidemia (abnormal amount of lipids in the blood). In periodontitis, an aberrant lipid metabolism can occur, elevating levels of low-density cholesterol and reducing levels of high-density cholesterol.8 This imbalance between the “bad” and “good” cholesterol commonly occurs in patients with ACVD and is widely prevented with medication.
The aforementioned mechanisms may occur separately, but most likely they happen simultaneously in patients with periodontitis and may increase the likelihood of atherogenesis. Atherogenesis results in atherosclerosis, which leads to endothelial dysfunction, arterial stiffness, reduction of blood vessel lumen size, high blood pressure, and, ultimately, ischemic events such as MI, cerebrovascular accident, and ACVD-related death. The pathologic conversion of a normal elastic artery into an atherosclerotic and stiff blood vessel results in endothelial dysfunction.8
Recent findings show that periodontitis shares some genetic risk factors with ACVD.8,11–13 Complex diseases like ACVD, type 2 diabetes, and Crohn’s disease may have similar and overlapping causative genetic variants; this is termed “pleiotropy of complex diseases.” A highly increased risk for aggressive periodontitis and evidence for increased risk of chronic periodontitis are located in a similar genetic location as ACVD.8 It is likely that there are common pathophysiologic pathways for both ACVD and periodontitis.8
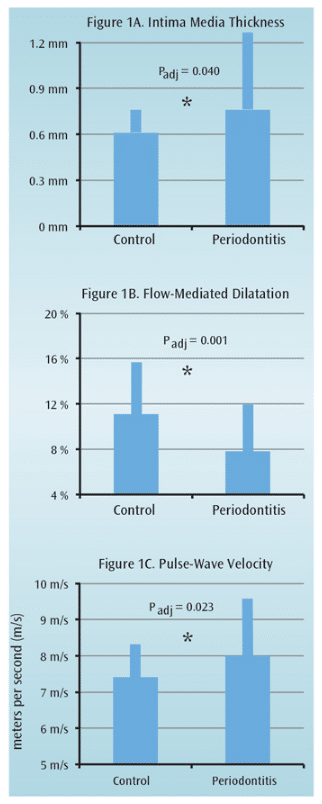
CLINICAL MARKERS OF ATHEROSCLEROSIS
The association between periodontitis and ACVD has been further evaluated by several clinical measures of atherosclerosis. The intima media thickness of the larger arteries (eg, carotids, brachial arteries) can be measured via ultrasound. An increase in intima media thickness has been shown to predict cardiovascular events, and measurements of carotid arteries are often used as surrogate clinical markers for determining the extent of atherosclerosis.14 The intima media thickness of the carotid arteries in relation to periodontitis has been assessed in 19 studies, 11 of which found a higher measurement of intima media thickness in periodontitis patients.1 For example, an increased measurement of intima media thickness was found in otherwise healthy periodontitis patients (mean age 51) compared to healthy nonperiodontitis patients (mean age 51); this was especially apparent in the internal carotid arteries (Figure 1A).15 This increased intima media thickness remained significant after adjusting for potential confounding factors such as age, gender, weight, cholesterol levels, and blood pressure. After statistical adjustments for potential confounding factors, the reported association between periodontitis and increased intima media thickness remained significant in nine studies.1
Flow-mediated dilatation is another noninvasive surrogate atherosclerotic parameter that evaluates potential endothelial dysfunction. It measures the percentage of dilatation of the brachial artery in response to pharmacological and physiological stimuli.16 For this measurement, the ability of the brachial artery to expand is recorded after the forearm and hand have been deprived of arterial blood by strong compression for 5 minutes. Six case-control studies evaluated flow-mediated dilatation in relation to periodontitis.1 Nearly all studies reported a significant endothelial dysfunction in periodontitis patients based on flow-mediated dilatation recordings. A relatively large variation in endothelial function, however, was detected. One of the first studies,17 reported only 8% dilatation capacity for otherwise healthy periodontitis patients, vs 11% in healthy controls (Figure 1B). This was a significant reduction in patients with periodontitis.
Recently, the relationship between arterial stiffness, as assessed by pulse-wave velocity, and ACVD has been investigated. Pulse-wave velocity is a reproducible noninvasive way to measure large artery stiffness and has emerged as a novel surrogate clinical marker for ACVD.18 Arterial stiffness provides a look at arterial function. Pulse-wave velocity has also been suggested in a pilot study as a clinical parameter for the prediction of cardiovascular mortality and morbidity, independent of traditional cardiovascular risk factors.19 Two cross-sectional studies determined pulse-wave velocity in individuals without severe comorbidities with and without periodontitis and without adjusting for potential confounding factors. The authors found a higher arterial stiffness in subjects with periodontitis.20,21 In a Japanese population, the prevalence of severe periodontitis was significantly higher in individuals with a pulse-wave velocity ?14 meters per second (m/s), compared to subjects with a lower pulse-wave velocity. After adjusting for potential confounding factors, no significant difference remained.21 A recent study conducted by researchers in the Department of Periodontology at the Academic Center for Dentistry Amsterdam (ACTA) measured pulse-wave velocity in subjects with periodontitis who were otherwise healthy (n=57, mean age 47) and compared the measurements to a control group (n=48, mean age 46).22 The patients with periodontitis exhibited a significantly higher pulse-wave velocity compared to the control group (Figure 1C). After adjusting for ACVD risk factors—including age, sex, smoking history, systolic blood pressure, and high cholesterol levels—the increased pulse-wave velocity in periodontitis remained significant.
As described above, the association between periodontitis and atherosclerosis has been evaluated by several indirect clinical measures of atherosclerosis: intima media thickness, flow-mediated dilatation, and pulse-wave velocity. These techniques evaluated carotid arteries, the brachial artery, and total main artery conditions, respectively. The general pattern emerging from these clinical studies is that vasculature health in patients with periodontitis is compromised compared to controls without periodontitis—even after adjusting for potential confounders, such as smoking, cholesterol levels, blood pressure, age, and gender.
EFFECT OF PERIODONTAL TREATMENT ON THE CARDIOVASCULAR SYSTEM
Given the associations of arterial stiffness and endothelial dysfunction with periodontitis, the question of whether cardiovascular health is improved after treatment of periodontitis must be asked. In other words, are there changes in the levels of endothelial dysfunction or arterial stiffness after basic periodontal therapy (scaling and root planing)? The body of evidence is small, but, in general, studies have shown that patients with periodontitis who receive nonsurgical periodontal therapy experience improvement in arterial function and/or reduced severity of atherosclerosis.1,23
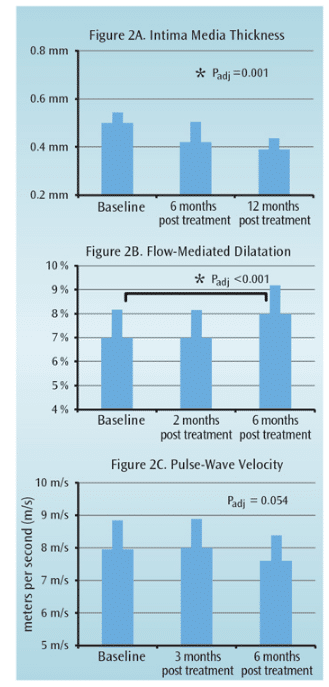
Two studies noted no difference in intima media thickness between baseline and 3 months after periodontal therapy.24,25 However, a longer follow-up at 6 months and 12 months revealed a significant reduction in intima media thickness compared to baseline (Figure 2A).25 In this study, 35 otherwise healthy subjects with periodontitis (mean age 46) received basic periodontal therapy. It is likely that periodontal therapy can improve the condition of the carotid artery. Notably, the regeneration rate in the intima-medial layer is relatively slow and may only be clinically detectable at 6 months post-treatment.
Six studies utilizing flow-mediated dilatation to measure endothelial function suggested a positive effect of periodontal therapy on flow-mediated dilatation at 4 weeks to 28 weeks post-surgery.1 Tonetti et al26 published a landmark paper in the New England Journal of Medicine that reported the results of periodontal treatment on the cardiovascular system, using flow-mediated dilatation of the brachial artery as a clinical outcome measure. The authors observed a significant improvement of the flow-mediated dilatation at 6 months, but not at 2 months, post-therapy (Figure 2B). Moreover, the flow-mediated dilatation at 6 months in patients with periodontitis who had received treatment was significantly better than the flow-mediated dilatation in the patients with periodontitis in the control group who obtained only community dental care. Improvement in flow-mediated dilatation follows the same model as intima media thickness—positive results take time.26 This phenomenon must be considered when evaluating studies with a relatively short follow-up (less than 6 months).
The effect of basic periodontal therapy on arterial stiffness via pulse-wave velocity has been examined in 47 patients without comorbidities, such as diabetes, ACVD, or morbid obesity, but with severe periodontitis.22 The pulse-wave velocity at baseline measured 8.00 m/s. After 3 months of therapy, the pulse-wave velocity increased slightly (8.14 m/s), but 6 months after the intervention, the pulse-wave velocity was reduced to 7.82 m/s (Figure 2C). This reduction in arterial stiffness failed to reach clinical significance, but corroborated the above mentioned improvements of endothelial function measured by intima media thickness and flow-related dilatation. Basically, periodontal therapy modestly improves the vascular condition. The improvements in the vascular system are not immediate, becoming measurable 6 months post-treatment. Whether these vascular improvements result in a reduction of ACVD events, however, is unknown.
Periodontal therapy may reduce the negative atherosclerotic changes in the vasculature, possibly improving endothelial function.1,23 Although the number of well-controlled, long-term studies is small, and no meta-analyses are available, it can be concluded from a recent systematic review23 that the effect of nonsurgical periodontal therapy on endothelial function is positive.
ROLE OF C-REACTIVE PROTEIN
The host responds to periodontal infection with a variety of responses involving both innate and adaptive immunity. It is thought that chronic periodontitis causes the elevation of several proteins, including interleukins 1, 6, 8, and tumor necrosis factor. Also, some coagulation-related biomarkers become elevated, such as fibrinogen and plasminogen activator inhibitor-1.
Although periodontitis is chronic in nature, low levels of acute-phase reactants are also produced in the liver among individuals with periodontitis, which demonstrates that periodontitis causes systemic inflammation.8–10 The acute-phase reactants have pro-inflammatory properties: they activate complement factors, neutralize invasive pathogens, and stimulate repair and regeneration of a variety of tissues.
C-reactive protein (CRP) appears to be an important biomarker of atherosclerosis. Chronic and slightly elevated levels of CRP (>3 mg/L) constitute risk predictors for ACVD.9 CRP is also currently regarded as a biomarker of systemic inflammation with the potential to increase the risk for ACVD. It is conceivable that elevated levels of CRP in periodontitis explain, at least in part, the association between periodontitis and ACVD.8
Many studies have examined the cross-sectional relationship between periodontitis and CRP. A systematic review, as well as meta-analyses and additional studies have shown convincing evidence that CRP is consistently elevated in patients with periodontitis compared to healthy controls.8,9 CRP is a nonspeci?c marker of the acute-phase response. In other words, many potential stimuli, including chronic infections and/or inflammatory conditions, smoking history, obesity, and trauma, may also account for mild increases in CRP. Therefore, the majority of studies have performed statistical adjustments for these potential confounders. Interestingly, many studies have found that CRP levels in patients with periodontitis are often above acritical level of 3.0 mg/L, up to 9 mg/L.9 As mentioned, elevated levels of CRP are associated with a higher incidence of acute thrombotic events, including stroke and MI, and may be linked to a chronic procoagulant state. As such, CRP levels may serve as surrogate markers for an increased long-term risk of ACVD.23
Systematic reviews and the most recent meta-analyses on CRP levels after completion of periodontal therapy demonstrate that scaling and root planing in patients with untreated periodontitis can reduce systemic CRP levels at 3 months and 6 months post-therapy.9,23,27 Several of the intervention trials that showed a decrease in CRP levels after basic periodontal therapy were completed using study populations with comorbidities (such as diabetes, overweight, smoking, and existing ACVD).9,23,27
The effect of basic periodontal therapy on CRP was reported in meta-analyses. Such analyses are based on randomized controlled trials with both treated (experimental group) and untreated periodontitis patients (control group), and the weighted mean difference (WMD) and associated confidence intervals (CI) between the treated group and the untreated control group were calculated. Negative values for WMD (and CI) indicate a decrease in CRP levels.
Teeuw et al23 reported a WMD of -0.50 mg/L (confidence interval [C1]: -0.78; -0.22) for CRP based on a meta-analysis that included all qualified studies (Figure 3). Notably, subanalyses showed that patients with periodontitis and any comorbidity benefited the most from periodontal therapy. Interestingly, the subanalyses, including randomized controlled trials, with otherwise healthy periodontitis patients showed CRP reductions that failed to reach statistical significance. This can be explained by the limited number of studies included in this particular subanalysis, as well as the limited elevation of CRP in patients with periodontitis but without comorbidities. Figure 3 provides a summary of the aggregated WMDs for CRP among all studies and subanalyses. Because smoking and being overweight are major risk factors for CVD, Teeuw et al23 performed a subanalysis on CRP levels by differentiating studies based on smoking habits and body mass index (BMI) of the study population. It appeared that nonsmokers benefited the most from periodontal therapy, whereas study populations consisting of both smokers and nonsmokers did not show a significant difference between the groups that received periodontal treatment and those that did not.
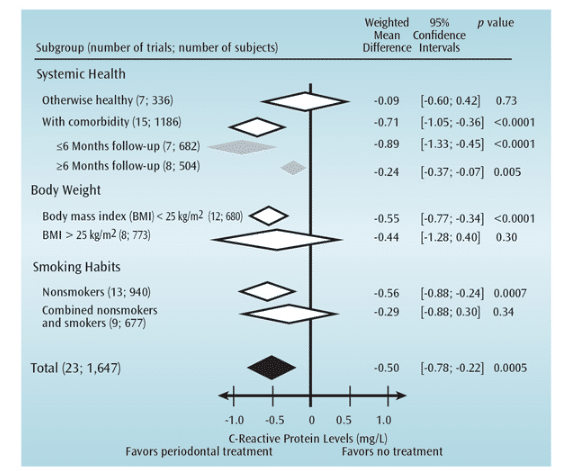
Reprinted with permission from: Teeuw WJ, Slot DE, Susanto H, et al. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta-analysis. J Clin Periodontol. 2014;41:70–79.
Similar results were obtained by differentiating studies according to the average BMI of the study population.23 Trials that included study subjects with normal weight showed CRP levels had a WMD of -0.55 mg/L (C1: -0.77; -0.34), while trials with an overweight study population showed CRP levels with a nonsignificant WMD of -0.44 mg/L (C1: -1.28; -0.40).
In trials that recruited patients with periodontitis and cardiovascular and/or metabolic disease (eg diabetes), differences in WMD between studies with different follow-up periods were examined. For example, several trials that lasted fewer than 6 months showed CRP levels with a WMD of -0.89 mg/L (C1: -1.33; -0.45), while another set of trials with a follow-up greater than 6 months showed CRP levels with a WMD of -0.24 mg/L (C1:?-0.37; -0.07). This suggests that the positive effect of periodontal therapy on reducing CRP levels may slowly subside in patients with comorbidities. Obviously, regular periodontal maintenance and follow-up care are needed.
In summary, many studies have investigated blood levels of CRP in relation to periodontitis. CRP is an important biomarker of ACVD, and elevated CRP levels in patients with periodontitis may explain the epidemiological association between these two conditions. CRP levels in patients with periodontitis are often elevated compared to healthy controls, and periodontal treatment studies have shown that basic nonsurgical periodontal therapy can reduce CRP levels below the critical plasma level of 2.1 mg/L. Patients with periodontitis who have existing comorbidities benefit most from periodontal intervention based on CRP reduction. Patients with periodontitis who smoke and/or are overweight will benefit most from their periodontal therapy if they also begin smoking-cessation and weight-loss efforts.
CONCLUSION
Periodontal diseases and ACVD are associated; however, the degree of this association on the development of ACVD is unclear. Several explanations for this relationship have been proposed: the presence of a pro-inflammatory state (eg, elevated CRP levels); presence of a pro-thrombotic state; elevated levels of immunological activity and auto-antibodies; and elevated cholesterol levels. These situations most likely manifest in concert, increasing endothelial dysfunction and the probability of an endothelial lining rupture in an atherosclerotic lesion and blood clot formation. As such, maintaining oral health is highly important.
The completion of periodontal therapy reduces the risk of bacteremia and improves the health of the vascular system. It also reduces systemic CRP levels—most prominently in those individuals with periodontitis who also have comorbidities, are of normal body weight, and do not smoke.
On the basis of these observations and in agreement with a recent consensus report of the joint EFP/AAP Workshop on Periodontitis and Systemic Diseases,6 cardiologists, endocrinologists, and general physicians should encourage their patients to be screened by dental professionals for the presence of periodontitis. Individuals with periodontitis should undergo periodontal therapy to improve their cardiovascular risk profile, thereby possibly reducing the risk of ACVD events. Additionally, dental professionals should discuss ACVD risk factors with their patients as part of their periodontal treatment protocol.
REFERENCES
- Han Y, Houcken W, Loos BG, Schenkein HA, Tezal M. Periodontal disease, atherosclerosis, adverse pregnancy outcomes, and head-and-neck cancer. Adv Dent Res. 2014;26:47–55.
- Humphrey LL, Fu R, Buckley DI, Freeman M, Helfand M. Periodontal disease and coronary heart disease incidence: a systematic review and meta-analysis. J Gen Intern Med. 2008;23:2079–2086.
- Dietrich T, Jimenez M, Krall Kaye EA, Vokonas PS, Garcia RI. Age-dependent associations between chronic periodontitis/edentulism and risk of coronary heart disease. Circulation. 2008;117:1668–1674.
- Friedewald VE, Kornman KS, Beck JD, et al. The American Journal of Cardiology and Journal of Periodontology editors’ consensus: periodontitis and atherosclerotic cardiovascular disease. J Periodontol. 2009;80:1021–1032.
- Dietrich T, Sharma P, Walter C, Weston P, Beck J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J Clin Periodontol. 2013;40(Suppl 14):S70–S84.
- Tonetti MS, VanDyke TE, Working group 1 of the joint EFP/AAP workshop. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013;(Suppl 14):S24–S29.
- de Oliveira C, Watt R, Hamer M. Toothbrushing, inflammation, and risk of cardiovascular disease: results from Scottish Health Survey. BMJ. 2010;340:c2451.
- Schenkein HA, Loos BG. Inflammatory mechanisms linking periodontal diseases to cardiovascular diseases. J Clin Periodontol. 2013;40(Suppl 14):S51–S69.
- Paraskevas S, Huizinga JD, Loos BG. A systematic review and meta-analyses on C-reactive protein in relation to periodontitis. J Clin Periodontol. 2008; 35:277–290.
- Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol. 2005;76(Suppl 11):2106–2115.
- Schaefer AS, Richter GM, Groessner-Schreiber B, et al. Identification of a shared genetic susceptibility locus for coronary heart disease and periodontitis. PLoS Genet. 2009;5:e1000378.
- Schaefer AS, Richter GM, Dommisch H, et al. CDKN2BAS is associated with periodontitis in different European populations and is activated by bacterial infection. J Med Genet. 2011;48:38–47.
- Schäfer AS, Bochenek G, Jochens A, et al. Genetic Evidence for PLASMINOGEN as a Shared Genetic Risk Factor of Coronary Artery Disease and Periodontitis. Circ Cardiovasc Genet. 2014 Dec 2. Epub ahead of print.
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–636.
- Leivadaros E, van der Velden U, Bizzarro S, et al. A pilot study into measurements of markers of atherosclerosis in periodontitis. J Periodontol. 2005;76:121–128.
- Anderson TJ. Arterial stiffness or endothelial dysfunction as a surrogate marker of vascular risk. Can J Cardiol. 2006;22(Suppl B):72B–80B.
- Amar S, Gokce N, Morgan S, Loukideli M, Van Dyke TE, Vita JA. Periodontal disease is associated with brachial artery endothelial dysfunction and systemic inflammation. Arterioscler Thromb Vasc Biol. 2003;23:1245–1249.
- Graham I, Atar D, Borch-Johnsen K, et al. European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts). Eur J Cardiovasc Prev Rehabil. 2007;14(Suppl 2):S1–113.
- Vlachopoulos C, Manesis E, Baou K, et al. Increased arterial stiffness and impaired endothelial function in nonalcoholic fatty liver disease: a pilot study. Am J Hypertens. 2010;23:1183–1189.
- Jockel-Schneider Y, Harks I, Haubitz I, et al. Arterial stiffness and pulse wave reflection are increased in patients suffering from severe periodontitis. PLoS One. 20141;9:e103449.
- Miyaki K, Masaki K, Naito M, et al. Periodontal disease and atherosclerosis from the viewpoint of the relationship between community periodontal index of treatment needs and brachial-ankle pulse wave velocity. BMC Public Health. 2006;14;6:131.
- Houcken W, Teeuw WJ, Bizzarro S, et al. Arterial stiffness is associated with periodontitis. Submitted for publication to J Hum Hypertens.
- Teeuw WJ, Slot DE, Susanto H, et al. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta-analysis. J Clin Periodontol. 2014;41:70–79.
- Seinost G, Wimmer G, Skerget M, et al. Periodontal treatment improves endothelial dysfunction in patients with severe periodontitis. Am Heart J. 2005;149:1050–1054.
- Piconi S, Trabattoni D, Luraghi C, et al. Treatment of periodontal disease results in improvements in endothelial dysfunction and reduction of the carotid intima-media thickness. FASEB J. 2009;23:1196–1204.
- Tonetti MS, D’Aiuto F, Nibali L, et al. Treatment of periodontitis and endothelial function. New Engl J Med. 2007;356:911–920.
- D’Aiuto F, Orlandi M, Gunsolley JC. Evidence that periodontal treatment improves biomarkers and CVD outcomes. J Clin Periodontol. 2013;40(Suppl 14):S85–S105.
From Dimensions of Dental Hygiene. February 2015;13(2):47–52.



