
Team Approach
There is no standard treatment for dentinal hypersensitivity (DH), but the first step toward effectively managing DH is preventing additional dentinal exposure or “resensitization” of asymptomatic dentin
ezcol_1third]
PURCHASE COURSE
This course was published in the Septemeber 2011 issue and expires September 2014. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss strategies for preventing additional dentin exposure.
- Define the watchful waiting with stimulus avoidance approach.
- Identify the importance of stabilizing pulpal tissues.
- Explain the roles of narrowing or occluding dentinal tubules and covering exposed tubular lumina in reducing dentinal hypersensitivity.
[/ezcol_1third]
There is no standard treatment for dentinal hypersensitivity (DH), but the first step toward effectively managing DH is preventing additional dentinal exposure or “resensitization” of asymptomatic dentin.1 Etiologies that expose dentin include: gingival recession, periodontitis, coronal enamel fractures, caries, erosion, abrasion, attrition, and abfraction. These are the manifestations of complex and highly individualized interactions that may be caused by disease, habits, or functional disorders.
Therefore, determining why symptomatic dentin became exposed and mitigating such etiologies are necessary in the prevention of additional dentinal exposure. For example, patients with DH provoked by aggressive toothbrushing may avoid brushing sensitive sites. Unfortunately, this can lead to inadequate self-care that may contribute to progressive gingival recession and additional exposure of root dentin. In such a case, the aggressive selfcare must be addressed in addition to DH. In the same context, a history of DH should spur careful patient questioning to determine where DH has been experienced so the practitioner can avoid exacerbating DH by limiting debridement or polishing of previously symptomatic root surfaces whenever possible.
Watchful Waiting with Stimulus Avoidance
Grossman first listed the components of ideal treatment for DH in 1935 as rapid acting with long-term relief, painless, easy to apply nonstaining, and not damaging to the pulp.2 In the absence of reliable treatments for DH, watchful waiting with stimulus avoidance was a common approach because most sites gradually improve if left alone. Not surprising, patients experiencing DH generally avoid cold or acidic beverages such as fruit juices, soft drinks, and even tea or coffee.3 Watchful waiting with stimulus avoidance remains a common self-care approach and sometimes makes good sense under professional guidance, especially when combined with one or more of the contemporary approaches that may not produce immediate or complete relief. In addition, patients may consume over-the-counter analgesics such as ibuprofen (not exceeding recommended dosages) as needed until symptoms decrease or cease.4
Stabilize Nociceptive Tissues
Nociceptive tissues have electrically-charged cell membranes that, when sufficiently provoked, produce action potentials that transmit nervous impulses to the brain. Stabilizing the ionic membrane potentials of such tissues results in partial or complete local anesthesia.
Potassium ions in aqueous solution appear to stabilize nociceptive cell membranes in a depolarized state by replacing smaller and naturally occurring sodium ions, thereby blocking nervous impulses that would otherwise produce pain. A dentifrice containing potassium nitrate was first introduced in the late 1970s, and was soon in widespread use. Competing topical agents soon added this substance to their formulations.
Potassium salts remain a component of numerous topical products thought useful for managing DH, although supporting evidence for this approach has not been considered robust in the past.5 However, a newly introduced potassium nitrate dentifrice (Colgate® Sensitive Pro-Relief™ Toothpaste with Enhanced Potassium Nitrate Technology) has demonstrated improved performance.6
Narrowing/Occluding Dentinal Tubules
Therapies that mimic or amplify natural desensitization processes produce effective outcomes. In recent decades, such professional and over-the-counter desensitizing products are increasingly ubiquitous and include three general subcategories: ion/salt compounds, protein precipitates, and resins. Some approaches outlined have been used for decades; others are relatively new (Table 1).
A number of surgical lasers in various wavelengths also show promise in the narrowing or occluding of dentinal tubules through the near-instantaneous melting of superficial peritubular dentin.7,8 However, pulpal effects remain under investigation,9-11 a placebo effect has been reported,12 and it is unclear whether laser ablation provides better results than other therapies.13
Topical silver nitrate has a long history in medicine as a disinfectant and hemostatic agent. It was initially used to manage DH when conveniently available formulations used for other purposes (eg, formaldehyde, glutaraldehyde, cavity varnishes) were empirically administered to patients with DH. Silver nitrate denatures native and bacterial proteins and can cause partial or complete cell necrosis. A dry mixture of 75% silver nitrate and 25% potassium nitrate impregnated into a cotton-tipped swab burnished onto root surfaces was often suggested to desensitize exposed dentin. Whether this now-obsolete treatment was a placebo remains in question,14 however, this treatment also exposed dentinal tubules to potassium ions, later recognized as potentially effective against DH. Also of note is that silver ions rapidly permeate tubules15 and produce severe staining.
Aluminum chloride is another treatment used in the past.16 Calcium hydroxide pastes have also been used with reported success.17 Strontium chloride was first added to a dentifrice about a half-century ago.18 Incompatible with fluoride-containing dentifrices, strontium chloride has been replaced by strontium acetate and appears effective,19 however, the benefit of strontium ions for DH treatment was recently questioned.20 Dibasic sodium citrate has shown minimal effectiveness.20 Sodium fluoride, stannous fluoride, sodium monofluorophosphate, and fluorosilicates—alone or combined with other agents—have been used with reported success for many years. Clinical trials22,23 have shown the ability of fluoride ions to decrease DH by precipitation of partially insoluble fluoride salts inside dentinal tubules.24 Enhancements such as iontophoresis25 and acidic carriers have been used to encourage crystal formations deep inside dentinal tubules. Oxalate-containing compounds can also form precipitates in dentinal tubules.26 For those who favor “natural” treatments, a mint, spinach, and rhubarb-sourced phytocomplex paste containing 5% potassium oxalate has been shown to occlude dentinal tubules in vitro.27 Numerous dentin sealers, adhesives, varnishes, and resins, alone or in combination, have also been used to successfully manage DH.28-30 Methylmethacrylate resin combined with gluteraldehyde can also be effective.31 Calcium silicate, calcium phosphate, amorphous calcium phosphate, and ammonium hexaflurosilicate compounds are also effective.32-35
The concept of combining clinical and self-care approaches is not new. Indeed, the use of potassium-ion containing dentifrices has long been combined with clinical applications of one or more of the narrowing/occluding technologies. However, two new product lines have been introduced that seek to synergize home and in-office delivery of select proprietary tubule-occluding compounds. 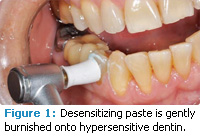
>The first new approach36 is built on the effectiveness37 of an arginine/calcium carbonate professional treatment (Colgate Sensitive Pro-Relief Densitizing Paste with Pro-Argin Technology), as well as a daily-use fluoride dentifrice that also contains arginine/calcium carbonate (Colgate Sensitive Pro-Relief Toothpaste with Pro-Argin Technology, not yet available in the United States). This approach uses the positive end of nondissociated amino-acid arginine molecules to form hydrogen bonds with exposed dentin to establish a higher pH intratubular environment.
This more alkaline environment accelerates the deposit of calcium carbonate and endogenous calcium ions within the dentinal tubular lumina. Other than the proven benefits of exogenous fluoride, this latter approach uses an amino acid common to the mouth and doesn’t require the use of chemicals alien to the oral environment (eg, glutaraldehyde, etching acids). In-office application immediately plugs dentinal tubular lumina (Figure 1), while daily use of the dentifrice continues to amplify and sustain natural tubular occlusive processes over time. Numerous studies suggest prompt and sustained effectiveness.38,39
The second approach includes the use of a prophylaxis paste containing calcium sodium phosphosilicate and fluoride (NUPRO® Sensodyne® Prophylaxis Paste, DENTSPLY Professional/GlaxoSmithKline) combined with a like-formulated dentifrice (Sensodyne NUPRO Professional Toothpaste), aimed at spurring the formation of hydroxyapatite-like crystals to occlude dentinal tubules. One study revealed relief from DH after 4 weeks that was superior to that provided by a dentifrice containing 5% potassium nitrate.40
Covering Exposed Lumina
Brännström’s widely accepted hydrodynamic theory of DH posits that noxious stimuli abruptly change normal tissue fluid flow within exposed dentinal tubules in vital teeth, thereby stimulating pain receptors that result in DH.41 Therefore, covering the external lumina of exposed dentinal tubules is a useful approach for managing DH.
Lumina coverage can be achieved by placing an indirectly fabricated and cemented restoration over the sites of exposed dentin. This approach is often the treatment of choice for dentin once covered by enamel (eg, restoratively replacing a fractured cusp). The technique of covering root dentin with cemented restorations has gained new life in recent years as a side-benefit of advancing technology associated with finely crafted veneers and crowns. Unfortunately, successful bonding of ceramics to dentin is highly technique sensitive,42 and single applications may be less than ideal when it comes to mitigating DH.43
In the early 1970s, polymeric restorative systems that no longer necessitated conventional cavity preparations were introduced. A short application of strong acid to create microscopic crystalline undercuts in enamel was followed by application of an unpolymerized resin flowed into the etched surfaces. Polymerization followed. The potential usefulness of this concept for treating DH didn’t go unrecognized. However, because dentin contains a smaller proportion of mineralized matrix than enamel, similar capabilities aimed at covering exposed dentinal tubules developed more slowly. In the meantime, the United States Food and Drug Administration accepted fluoride varnishes for managing DH.44 Although fluoride varnishes remain useful in this context, glass ionomer-based methodologies may be at the forefront of luminal covering technologies because ionomers are longer-lived than varnishes, other dentin sealers, or polymeric adhesives.45
Glass ionomers, initially developed as dental cements, are unique materials because they self-adhere via ionic bonds to calcium molecules in the mineralized matrices of both enamel and dentin. Furthermore, glass ionomers gradually release fluoride ions, providing a caries inhibition feature that can be continually recharged by exposure to fluoride dentifrices, fluoridated water, and professionally applied fluoride treatments. Years of incremental improvements have resulted in a host of newer products aimed at making glass ionomers more versatile, easier to use, increasingly esthetic, and wear-resistant. However, where optimal esthetic results or better wear-resistance are required, veneers or composite resins applied to acid-etched dentin are sometimes preferred.
Covering DH-causing root surfaces with gingival tissue is often a useful clinical approach and is frequently used in combination with other treatments. Indeed, treating DH is one of the three generally accepted indications for gingival grafting. The remaining two are: to improve esthetics, and to provide an anatomy more resistant to continuing gingival recession. Especially in anterior areas of the mouth, restoring lost tissue can recreate an anatomy closer to what existed originally. Occasionally, notched root dentin at cervical areas with limited gingival recession can be completely restored by tissue grafting, thereby optimizing esthetics while eliminating DH. Unfortunately, at sites where root exposure is broad, or where interproximal tissue losses have occurred, the amount of root surface that can be covered is more limited.
Management Factors
Other than determining why dentin was exposed and preventing additional exposure, appropriate management of DH may vary. Patient preferences are important, and patients should be informed of the possibility that DH may recur and require follow-up therapy. Some patients may prefer to simply “tough it out” and rely on nature to gradually mitigate their DH. Others may be content with interventions that show improvements over weeks or months, while others may seek immediate relief. Table 2 lists the key points that patients need to understand about DH treatment. Patients usually respond happily to results that exceed their expectations, and unhappily to promises that aren’t achieved. Although a placebo effect is often discounted, it can be an important ally in helping achieve and sustain pain relief.
Recommending the use of a potassiumion-containing gel or dentifrice in combination with watchful waiting with stimulus avoidance has long been the favored initial approach for DH management. However, many patients seek immediate improvement, or prefer to avoid the occasional discomfort associated with the use of potassium- or fluorideion-containing compounds. For these patients, faster-acting professional approaches aimed at narrowing or occluding dentinal tubules make good sense, especially in the context of minimal cost difference. Iontophoresis-based acceleration of tubular narrowing or occlusion46 followed by resin impregnation (or longer lasting45) glass ionomer coverage will usually provide both immediate and lasting relief. Fluoride varnish is easy to apply and can also provide prompt relief. Furthermore, as fluoride varnish gradually erodes, an incremental and symptom-free transition to naturally occurring tubular narrowing/occluding may take place.
Because numerous approaches to narrowing/occluding dentinal tubules appear effective, weighing the speed of relief, cost, patients’ desires, and professional preferences must all be considered. Although unproven, it seems likely that approaches which partner both the practitioner and patient in synergistic efforts may be best because a reinforcing and enthusiastic team approach may produce a stronger placebo effect as well.47
>When more than one management goal can be achieved, the choice of more costly and involved approaches to managing DH may make sense. For example, if loss of enamel or gingival recession is the cause of the dentinal exposure, procedures aimed at permanently covering the tubules may be the best choice to successfully treat DH while removing the etiology of the dentinal exposure and simultaneously re-establishing optimal oral function and esthetics.
Concluding Remarks
Watchful waiting with stimulus avoidance, stabilizing nociceptive tissues, narrowing/occluding dentinal tubules, and covering tubular lumina are common approaches to managing DH. Clinical lasers that instantaneously ablate dentinal lumina also show promise. However, regardless of what interventions are selected, practitioners need to determine the cause of dentin exposure and mitigate it if it is ongoing. Although DH tends to improve no matter what therapy is provided, interventions that team the patient and the dental professional are most likely to provide the best results. New treatments for DH, including combined clinical and patientapplied therapies to occlude/narrow dentinal tubules, are effective options to help manage the painful problem of dentinal hypersensitivity.
Table 2. Key points for patient education
- There are numerous ways to manage DH.
- Although total relief can be achieved, DH recurrence is always a possibility.
- A combination of professional therapy and daily self-care is best.
- If one approach doesn’t work, try another.
REFERENCES
- Porto ICCM, Andrade AKM, Montes MAJR. Diagnosis and treatment of dentinal hypersensitivity. J Oral Sci. 2009;51:323-332.
- Grossman L. A systematic method for the treatment of hypersensitive dentine. J Am Dent Assoc. 1935;22:592-598.
- Lussi A, Jaeggi T. Chemical factors. Monogr Oral Sci. 2006;20:77-87.
- Hersh EV, Kane WT, O’Neil MG, et al. Prescribing recommendations for the treatment of acute pain in dentistry. Compend Contin Educ Dent. 2011;32:22, 24-30
- Markowitz K, Pashley DH. Discovering new treatments for sensitive teeth: the long path from biology to therapy. J Oral Rehabil. 2008;35:300-315.
- Delgado E, et al. Clinical efficacy of an enhanced potassium nitrate desensitizing toothpaste. Am J Dent. In review.
- Gholami GA, Fekrazad R, Esmaiel-Nejad A, Kalhori KAM. An evaluation of the occluding effects of Er;Cr:YSGG, Nd:YAG, COâ‚ and diode lasers on dentinal tubules: a scanning electron microscope in vitro study. Photomed Laser Surg. 2011;29:115-121.
- Badran Z, Boutigny H, Struillou X, et al. Tooth desensitization with an Er:YAG laser: in vitro microscopical observation and a case report. Lasers Med Sci. 2011;26:139-142.
- Lin M, Xu F, Lu TJ, Bai BF. A review of heat transfer in human tooth— experimental characterization and mathematical modeling. Dent Mater. 2010;26:501-513.
- Romano AC, Aranha AC, da Silveira BL, Baldochi SL, Eduardo Cde P. Evaluation of carbon dioxide laser irradiation associated with calcium hydroxide in the treatment of dentinal hypersensitivity. A preliminary study. Lasers Med Sci. 2011;26:35-42.
- Birang R, Poursamimi J, Gutknecht N, Lampert F, Mir M. Comparative evaluation of the effects of Nd:YAG and Er:YAG laser in dentin hypersensitivity treatment. Lasers Med Sci. 2007;22:21-24.
- Birang R, Kaviani N, Mohammadpour M, et al. Evaluation of Nd:YAG laser on partial oxygen saturation of pulpal blood in anterior hypersensitive teeth. Lasers Med Sci. 2008;23:291-294.
- Kara C, Orbak R. Comparative evaluation of Nd:YAG laser and fluoride varnish for the treatment of dentinal hypersensitivity. J Endod. 2009;35:971-974.
- Anderson DJ, Matthews B. An investigation into the reputed desensitizing effect of applying silver nitrate and strontium chloride to human dentine. Arch Oral Biol. 1966;11:1129-1135.
- Iwamoto N, Shimada Y, Tagami J. Penetration of silver nitrate into bleached enamel, dentin, and cementum. Quintessence Int. 2007;38:e183-188.
- Seto BG. Mechanism of systemic allergy: report of a case involving an aluminum chloride solution. J Oral Med. 1982;37:87-94.
- Zaimoglu A, Aydin AK. An evaluation of smear layer with various desensitizing agents after tooth preparation. J Prosthet Dent. 1992;68:450-457.
- Markowitz K. The original desensitizers: strontium and potassium salts. J Clin Dent. 2009;20:145-151.
- Parkinson CR, Willson RJ. An in vitro investigation of two currently marketed dentin tubule occlusion dentifrices. J Clin Dent. 2011;22:6-10.
- Cummins D. Recent advances in dentin hypersensitivity: clinically proven treatments for instant and lasting sensitivity relief. Am J Dent. 2010;23SpecNo A:3A-13A.
- Ong G, Strahan JD. Effect of a desensitizing dentifrice on dentinal hypersensitivity. Endod Dent Traumatol. 1989;5:213-218.
- Morris MF, Davis RD, Richardson BW. Clinical efficacy of two dentin desensitizing agents. Am J Dent. 1999;12:72-76.
- Leonard RH Jr, Smith LR, Garland GE, Caplan DJ. Desensitizing agent efficacy during whitening in an at-risk population. J Esthet Restor Dent. 2004;16:49-55, 56.
- Orchardson R, Gillam DG. Managing dentin hypersensitivity. J Am Dent Assoc. 2006;137:990-998.
- Kern DA, McQuade MJ, Scheidt MJ, Hanson B, Van Dyke TE. Effectiveness of sodium fluoride on tooth hypersensitivity with and without iontophoresis. J Periodontol. 1989;60:386-389.
- Pillon FL, Romani IG, Schmidt ER. Effect of a 3% potassium oxalate topical application on dentinal hypersensitivity after subgingival scaling and root planing. J Periodontol. 2004;75:1461-1464.
- Sauro S, Gandolfi MG, Prati C, Mongiorgi R. Oxalate-containing phytocomplexes as dentine desensitisers: an in vitro study. Arch Oral Biol. 2006;51:655-664.
- Prati C, Cervellati F, Sanasi V, Montebugnoli L. Treatment of cervical dentin hypersensitivity with resin adhesives: 4-week evaluation. Am J Dent. 2001;14:378-382.
- Baysan A, Lynch E. Treatment of cervical sensitivity with a root sealant. Am J Dent. 2003;16:135-138.
- Duran I, Sengun A. The long-term effectiveness of five current desensitizing products on cervical dentine sensitivity. J Oral Rehabil. 2004;31:351-356.
- Dondi dall’Orologio G, Lorenzi R, Anselmi M, Opisso V. Dentin desensitizing effects of Gluma Alternate, Health-Dent Desensitizer and Scotchbond Multi- Purpose. Am J Dent. 1999;12:103-106.
- Peacock JM, Orchardson R. Action potential conduction block of nerves in vitro by potassium citrate, potassium tartrate and potassium oxalate. J Clin Periodontol. 1999;26:33-37.
- Fiocchi MF, Moretti AJ, Powers JM, Rives T. Treatment of root sensitivity after periodontal therapy. Am J Dent. 2007;20:217-220.
- Geiger S, Matalon S, Blasbalg J, Tung M, Eichmiller FC. The clinical effect of amorphous calcium phosphate (ACP) on root surface hypersensitivity. Oper Dent. 2003;28:496-500.
- Suge T, Kawasaki A, Ishikawa K, Matsuo T, Ebisu S. Effects of ammonium hexafluorosilicate concentration on dentin tubule occlusion and composition of the precipitate. Dent Mater. 2010;26:29-34.
- Salian S, Thakur S, Kulkarni S, LaTorre G. A randomized controlled clinical study evaluating the efficacy of two desensitizing dentifrices. J Clin Dent. 2010;21:82-87.
- Petrou I, Heu R, Stranick M, et al. A breakthrough therapy for dentin hypersensitivity: how dental products containing 8% arginine and calcium carbonate work to deliver effective relief of sensitive teeth. J Clin Dent. 2009;20:23-31.
- Kleinberg I. SensiStat. A new saliva-based composition for simple and effective treatment of dentinal sensitivity pain. Dent Today. 2002;21:42-47.
- Schiff T, Delgado E, Zhang YP, et al. Clinical evaluation of the efficacy of an in-office desensitizing paste containing 8% arginine and calcium carbonate in providing instant and lasting relief of dentin hypersensitivity. Am J Dent. 2009;22(Spec No A):8A-15A.
- Hamlin D, Williams KP, Delgado E, et al. Clinical evaluation of the efficacy of a desensitizing paste containing 8% arginine and calcium carbonate for the in-office relief of dentin hypersensitivity associated with dental prophylaxis. Am J Dent. 2009;22(Spec No A):16A-20A.
- Brännström M. The hydrodynamics of the dental tubule and pulp fluid: its significance in relation to dentinal sensitivity. Annu Meet Am Inst Oral Biol. 1966;23:219.
- Vasconcellos WA, Alvim HH, Saad JRC, Susin AH. Effects of surface treatment on the microtensile bond strength of ceramic materials to dentin. Acta Odontol Latinoam. 2007;20(2):103-107.
- de Assis C de A, Antoniazzi RP, Zanatta FB, Rösing CK. Efficacy of Gluma Desensitizer on dentin hypersensitivity in periodontally treated patients. Braz Oral Res. 2006;20:252-256.
- Mandel ID. Fluoride varnishes—a welcome addition. J Public Health Dent. 1994;54:67.
- Polderman RN, Frencken JE. Comparison between effectiveness of a low viscosity glass ionomer and a resin-based glutaraldehyde containing primer in treating dentine hypersensitivity—a 25.2-month evaluation. J Dent. 2007;35:144-149.
- Singal P, Gupta R, Pandit N. 2% sodium fluoride-iontophoresis compared to a commercially available desensitizing agent. J Periodontol. 2005;76:351-357.
- Moerman DE, Jonas WB. Deconstructing the placebo effect and finding the meaning response. Ann Intern Med. 2002;136:471-476.
From Dimensions of Dental Hygiene. September 2011 Supplement; 9(9): 58-61.



