
Dentinal Hypersensitivity Defined
Sponsored by the Colgate-Palmolive Company, earn up to four CE units with this 16-page supplement that presents evidence-based strategies for the diagnosis and treatment of dentinal hypersensitivity.
This course was published in the Septemeber 2011 issue and expires September 2014. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Define dentinal hypersensitivity (DH).
- Discuss the prevalence of DH.
- Explain the pathophysiology and diagnosis of DH.
Gingival recession resulting in exposed root surfaces prone to DH is highly prevalent among those 65 years of age and older, and is more common among men than in age-matched women.7,8 The prevalence of DH in patients who have undergone periodontal treatment is also significant, perhaps as high as 80% to 90%.9
WHAT IS DENTINAL HYPERSENSITIVITY?
The dental pulp produces, repairs, maintains, and supplements dentin and detects noxious (harmful) stimuli. Mature primary dentin is composed of small-diameter tubules that radiate peripherally from the pulp and extend through the dentin’s mineralized matrix, ending at the dentin-enamel interface in tooth crowns or the dentin-cementum interface in roots (Figure 1). In healthy dentin unexposed to the oral cavity, dentinal tubules contain odontoblastic cell processes responsible for maintaining or repairing dentin’s organic and mineral matrices. These odontoblastic processes also play communicative roles in DH, and the cells themselves occasionally demonstrate the ability to respond to mechanical pressure and temperature changes.10,11 The neural response of dentin to noxious stimuli is both autonomic and nociceptive.11,12 The evolutionary purpose for dentin’s sensory responses is not clear, but there is little question that freshly exposed, vital dentin subjected to noxious stimuli produces pain in most people.
TERMINOLOGY
The term dentinal hypersensitivity has been used for many years to describe the normal sensitivity of freshly exposed vital dentin to noxious stimuli, which include thermal (typically evoked by cold beverages or salivary evaporation), tactile, chemical (including acids and whitening agents), and osmotic types.13-15 Cold is the most commonly reported stimulus.16
DH is most often experienced as transient pain that ends when the provoking stimulus is removed.17 However, it can occasionally manifest as a chronic condition characterized by acute episodes that subside substantially but incompletely following removal of the causative stimulus.18
PATHOPHYSIOLOGY
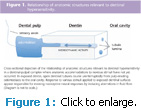 Dentin can become exposed to the oral environment in a variety of ways, such as gingival recession or loss of enamel via caries, fracture, abrasion, erosion, attrition, or abfraction. Some of these conditions may be caused by periodontitis, bruxism, or hyposalivation.19,20 In the case of newly exposed tooth roots, prompt loss of cementum tends to occur.21 Loss of enamel or cementum uncovers the lumina of the dentinal tubules (Figure 1). DH occurs when dentinal lumina are exposed to noxious stimuli of sufficient intensity. According to Bränström’s hydrodynamic theory of DH, noxious stimuli alter the normal, slow fluid flow within exposed dentinal tubules.22 Cold and tactile stimuli are thought to rapidly push tubular fluid toward the pulp, whereas heat is thought to draw fluid toward the mouth. In either case, rapid changes in the normal rates of tubular fluid flow appear to excite nociceptive receptors in the pulp.23
Dentin can become exposed to the oral environment in a variety of ways, such as gingival recession or loss of enamel via caries, fracture, abrasion, erosion, attrition, or abfraction. Some of these conditions may be caused by periodontitis, bruxism, or hyposalivation.19,20 In the case of newly exposed tooth roots, prompt loss of cementum tends to occur.21 Loss of enamel or cementum uncovers the lumina of the dentinal tubules (Figure 1). DH occurs when dentinal lumina are exposed to noxious stimuli of sufficient intensity. According to Bränström’s hydrodynamic theory of DH, noxious stimuli alter the normal, slow fluid flow within exposed dentinal tubules.22 Cold and tactile stimuli are thought to rapidly push tubular fluid toward the pulp, whereas heat is thought to draw fluid toward the mouth. In either case, rapid changes in the normal rates of tubular fluid flow appear to excite nociceptive receptors in the pulp.23
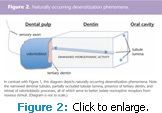
Over time, intra-tubular calcifications that narrow the dentinal tubules usually occur naturally (Figure 2). A proteinaceous and partially calcified smear layer will also develop on the surface of exposed dentin, and may partially or completely occlude the tubular lumina.24 Indeed, dentin prone to DH has a thinner, less calcified smear layer, more lumina per square millimeter, larger gauge lumina, and higher degrees of tubular patency.25 Furthermore, exposure of dentin to the oral cavity can provoke the slow formation of tertiary (sometimes called reactionary) dentin (Figure 2) inside the pulp. Amorphous tertiary dentin is deposited over the internal or pulp-side lumina of dentinal tubules and aids in limiting rapid changes in the flow rates of tubular fluid.26, 27
The processes discussed above and depicted in Figure 2 may explain why symptoms may not appear when dentin is gradually exposed to the mouth—a fairly common occurrence at sites where gingival recession occurs. This phenomenon supports the observation that tubular fluid flow is related to tubular diameter.28 However, research suggests that odontoblasts may share at least some properties long thought to be only the province of nerve cells.1 This evidence supports an alternative or additional hypothesis for DH that suggests the odontoblastic processes in the dentinal tubules possess inherent sensory characteristics, making them functional nociceptive transducers. This hypothesis reinforces the idea that natural desensitization processes also seek to better isolate odontoblasts from noxious stimuli. The suggestion that odontoblasts may serve as sensory cells is also indirectly supported by long-standing evidence that some desensitizing toothpastes function by stabilizing trans-cell-membrane ionic potentials—making sensory tissues less responsive to noxious stimuli. It is also plausible that numerous pathophysiological sequences, some as yet undiscovered, are responsible for DH.
DIAGNOSIS
Diagnoses of DH are not limited to the exposed root surfaces that characterize gingival recession. DH is often a “diagnosis by exclusion,” similar to medical problems with multiple causes, such as fibromyalgia.29 Accurate diagnosis of DH sometimes requires that practitioners dismiss other potential etiologies.30
DH typically involves myelinated rapid conducting nerve fibers, but it may also manifest through slower-conducting nonmyelinated nerves that are sometimes associated with dull, persistent, chronic, and even referred pain that can make it difficult to determine the origin of the pain.31,32 Dentinsourced pain may arise from a number of stimuli including thermal changes, improper toothbrushing,33 tooth whitening,34 and hyperocclusive disorders (eg, bruxism and clenching),35 as well as dietary,36 gastric,37 or atmospheric acids.38
The first and sometimes only step needed to diagnose DH is to locate and test the exposed dentin suspected as the source of symptoms. This is often quite simple. For example, a fractured cusp is frequently easy to detect and patients can finger the problematical tooth or teeth. When additional testing is needed to determine the source of DH, a brief blast of air (after isolating the suspected site) or gentle probing will usually evoke pain, thereby confirming DH while identifying its source. If a patient can’t pinpoint the source of sensation, diagnosis can be more difficult. For example, abfractive lesions may be responsible for DH despite minimal gingival recession. DH may also originate from one or more sites simultaneously.
Occasionally, no symptomatic exposed dentin can be located. Clinicians need to rule out other potential causes, including sinusitis, gingival allergic responses (eg, to certain dentifrices), tooth or restoration fracture (suddenly exposing dentin to the oral environment or to new mechanical stresses), and active caries or pulpal inflammation (pulpitis). A tooth with a new restoration may evoke symptoms that mimic DH. Fortunately, these symptoms usually diminish within days or weeks.
A fractured tooth or dental restoration, not uncommon in older adults, can sometimes be detected by visual examination. Often patients with this type of injury can reveal exactly what they were doing (eg, chewing ice) when the symptoms first appeared. Such anecdotes are key clues to the etiology of patients’ symptoms. Vertical fractures that extend into the root are difficult to see, but pain is readily felt when the patient occludes on the suspect tooth. Inserting a small gauge, soft wooden or plastic cotton swab handle between a suspect tooth and its opposite-arch counterpart, followed by a gentle attempt by the patient to occlude in both centric and eccentric intercuspal relations, is often a straightforward way to identify a tooth with a hidden fracture. Also, in the case of molar or premolar teeth, using the handle ends of two dental instruments to determine if a portion of a crown can be moved independently from the remainder of the crown can confirm a vertical fracture that might not otherwise be detected. A periodontal endoscope can also help detect a highly symptomatic but difficult-to-detect (complete or incomplete) vertical or oblique tooth root fracture. Unfortunately, conventional dental radiography, either digital or film-based, is typically useless in detecting subtle changes in radiopacities.39 Furthermore, small or internal fractures may cause pain but escape clinical detection.
Symptomatic teeth should be examined for signs of coronal and root caries. Caries incidence increases among older patients, likely resulting from natural or drug-induced changes in salivary flow or diet. Interproximal root caries can be especially difficult to detect and treat.40 Large or new restorations, including crowns or clinical signs of caries, are often associated with pulpitis that may or may not be reversible. A vital tooth’s increased sensitivity to a cold stimulus may mimic DH. Fortunately, these etiologies can usually be distinguished from DH that originates from exposed root dentin. Accurate diagnosis can often be successfully accomplished by isolating the suspected caries site or restoration from any exposed root surface site followed by independent evaluations of each site. Cold or heat stimuli can be applied to a tooth’s crown in an effort to gauge the tooth’s reaction compared with other analogous asymptomatic teeth in the patient’s mouth. Unlike teeth afflicted by DH, pulpitis-affected teeth often respond disproportionately to coronally applied cold or heat. Although areas of exposed root dentin are sometimes highly responsive to cold (typically evoked by moving air that causes quick cooling by evaporating saliva on the exposed root), teeth with pulpitis may be highly sensitive to cold, heat, or both.
And unlike DH, sometimes the acute discomfort caused by thermal testing in pulpitis-afflicted teeth can persist for some time after removal of the noxious stimulus. Radiographs are insensitive to subtle changes, but peri-apical bony radiolucencies may be related to irreversible pulpitis, especially in multi-rooted teeth that remain highly sensitive to cold or hot while the pulpal contents of each root undergo necrosis at different rates. Electronic pulp testing may also be useful in discriminating between potential causes of symptoms.
A thorough patient history may be helpful in assessment. Recent periodontal surgery may have exposed previously unexposed root surfaces prone to DH, especially during the immediate postoperative weeks or months. Abusive oral habits, including improper or compulsive toothbrushing, may play roles.4 Patients with exposed root surfaces on the facial aspects of teeth who report DH shortly after undergoing dental prophylaxis, scaling or root planing may have been over-instrumented so that previously occluded dentinal tubules are rendered open.
Patients with a history of DH at one or more sites are more likely to experience DH than those with no previous history. Other patients may complain of temporary and more widespread symptoms that suggest DH but are actually symptoms of gingival irritation. Rapidly progressing gingival recession at one or more sites in conjunction with DH symptoms is also a common finding. Frequent consumption of acidic (low pH) beverages, such as soft drinks, may also provoke DH from dentin that is otherwise symptom-free. Tooth whitening has been implicated in DH as well.41,42 Smoking has long been suspected as an etiology, but a recent study suggests otherwise.2 DH may arise from more than one cause. Finally, clinicians may occasionally be presented with situations where the source of the pain remains ambiguous despite rigorous attempts to isolate various causes. In such cases, it’s appropriate to engage in “watchful waiting” to see how symptoms develop over time or to institute inexpensive interventions, such as many described in the following article (Team Approach). Patients need to be made aware when a definitive diagnosis can’t be made.
SUMMARY AND CONCLUSION
Dentinal hypersensitivity is a term used to denote the normal sensitivity of exposed tooth root dentin mediated by fast-conducting, myelinated, afferent nerves from the dental pulp. Not all exposed dentin produces such sensations. DH is common, especially among patients who have recently undergone periodontal therapies that exposed previously unexposed root surfaces, as well as periodontal debridement patients who have undergone instrumenting of previously exposed root surfaces. A number of pathophysiological mechanisms for DH have been proposed based largely on observational and comparative studies contrasting the anatomic features of nonsensitive exposed dentin with symptomatic dentin. Diagnosis of DH is usually straightforward—however, when it’s not, practitioners must rule out other causes of a patient’s symptoms.
REFERENCES
- Clayton DR, McCarthy D, Gillam DG. A study of the prevalence and distribution of dentine sensitivity in a population of 17 58-year-old serving personnel on an RAF base in the Midlands. J Oral Rehabil. 2002;29:14-23.
- Que K, Ruan J, Fan X, Liang X, Hu D. A multi-centre and cross-sectional study of dentine hypersensitivity in China. J Clin Periodontol. 2010;37:631-637.
- von Troil B, Needleman I, Sanz M. A systematic review of the prevalence of root sensitivity following periodontal therapy. J Clin Periodontol. 2002;29(Suppl):173-177, 195-196.
- Bamise CT, Olusile AO, Oginni AO, Dosumu OO. The prevalence of dentine hypersensitivity among adult patients attending a Nigerian teaching hospital. Oral Health Prev Dent. 2007;5:49-53.
- Kehua Q, Yingying F, Hong S, et al. A cross-sectional study of dentine hypersensitivity in China. Int Dent J. 2009;59:376-380.
- Bamise CT, Kolawole KA, Oloyede EO, Esan TA. Tooth sensitivity experience among residential university students. Int J Dent Hyg. 2010;8:95-100.
- Gorman WJ. Prevalence and etiology of gingival recession. J Periodontol. 1967;38:316-322.
- Kassab MM, Cohen RE. The etiology and prevalence of gingival recession. J Am Dent Assoc. 2003;134:220-225.
- Chabanski MB, Gillam DG, Bulman JS, Newman HN. Prevalence of cervical dentine sensitivity in a population of patients referred to a specialist periodontology department. J Clin Periodontol. 1996;23:989-992.
- Magloire H, Maurin JC, Couble ML, et al. Topical review. Dental pain and odontoblasts: facts and hypotheses. J Orofac Pain. 2010;24:335-349.
- El Karim IA, Linden GJ, Curtis TM, et al. Human odontoblasts express functional thermo-sensitive TRP channels: Implications for dentin sensitivity. Pain. 2010 Dec 17. Epub ahead of print.
- Feinstein B, Langton JN, Jameson R, Schiller F. Experiments on pain referred from deep somatic tissues. J Bone Joint Surg Am. 1954;36-A:981-997.
- Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol. 1997;24:808-813.
- Christensen GJ. Desensitization of cervical tooth structure. J Am Dent Assoc. 1998;129:765-766.
- Browning WD, Blalock JS, Frazier KB, Downey MC, Myers ML. Duration and timing of sensitivity related to bleaching. J Esthet Restor Dent. 2007;19:256-264.
- Irwin CR, McCusker P. Prevalence of dentine hypersensitivity in a general dental population. J Ir Dent Assoc. 1997;43:7-9.
- Canadian Advisory Board on Dentin Hypersensitivity. Adapted from Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc. 2003;69:221-226.
- Curro FA. Tooth hypersensitivity in the spectrum of pain. Dent Clin North Am. 1990;34:429-437.
- Terry DA. Cervical dentin hypersensitivity: etiology, diagnosis and management. Dent Today. 2011;30:61-62, 64, 66.
- Orchardson R, Collins WJ. Clinical features of hypersensitive teeth. Br Dent J. 1987;162:253-256.
- Bevenius J, Lindskog S, Hultenby K. The micromorphology in vivo of the buccocervical region of premolar teeth in young adults. A replica study by scanning electron microscopy. Acta Odontol Scand. 1994;52:323-334.
- Brännström M. The hydrodynamics of the dental tubule and pulp fluid: its significance in relation to dentinal sensitivity. Annu Meet Am Inst Oral Biol. 1966;23:219.
- Rimondini L, Baroni C, Carrassi A. Ultrastructure of hypersensitive and non-sensitive dentine. A study on replica models. J Clin Periodontol. 1995;22:899-902.
- Bowen RL. Adhesive bonding of various materials to hard tooth tissues—solubility of dentinal smear layer in dilute acid buffers. Int Dent J. 1978;28:97-107.
- Absi EG, Addy M, Adams D. Dentine hypersensitivity. The development and evaluation of a replica technique to study sensitive and non-sensitive cervical dentine. J Clin Periodontol. 1989;16:190-195.
- Smith AJ, Murray PE, Sloan AJ, Matthews JB, Zhao S. Trans-dentinal stimulation of tertiary dentinogenesis. Adv Dent Res. 2001;15:51-54.
- Goldberg M, Kulkarni AB, Young M, Boskey A. Dentin: structure, composition and mineralization. Front Biosci (Elite Ed). 2011;3:711-735.
- Addy M. Dentine hypersensitivity: New perspectives on an old problem. Int Dent J. 2002;52(Suppl):367-375.
- Häuser W, Eich W, Herrmann M, et al. Fibromyalgia syndrome: classification, diagnosis, and treatment. Dtsch Arztebl Int. 2009;106:383-391.
- Miglani S, Aggarwal V, Ahuja B. Dentin hypersensitivity: Recent trends in management. J Conserv Dent. 2010;13:218-224.
- Abd-Elmeguid A, Yu DC. Dental pulp neurophysiology: part 1. Clinical and diagnostic implications. J Can Dent Assoc. 2009;75:55-59.
- Addy M. Etiology and clinical implications of dentine hypersensitivity. Dent Clin North Am. 1990;34:503-514.
- Addy M. Tooth brushing, tooth wear and dentine hypersensitivity—are they associated? Int Dent J. 2005;55(Suppl):261-267.
- Leonard RH Jr, Haywood VB, Phillips C. Risk factors for developing tooth sensitivity and gingival irritation associated with nightguard vital bleaching. Quintessence Int. 1997;28:527-534.
- Lehman ML, Meyer ML. Relationship of dental caries and stress: concentrations in teeth as revealed by photoelastic tests. J Dent Res. 1966;45:1706-1714.
- Cox CF. Etiology and treatment of root hypersensitivity. Am J Dent. 1994;7:266-270.
- Robb ND, Smith BG, Geidrys-Leeper E. The distribution of erosion in the dentitions of patients with eating disorders. Br Dent J. 1995;178:171-175.
- Amin WM, Al-Omoush SA, Hattab FN. Oral health status of workers exposed to acid fumes in phosphate and battery industries in Jordan. Int Dent J. 2001;51:169-174.
- Rethman M, Ruttiman U, O’Neal R, et al. Diagnosis of bone lesions by subtraction radiography. J Periodontol. 1985;56:324-329.
- da Silva Neto JM, dos Santos RL, Sampaio MCC, Sampaio FC, Passos IA. Radiographic diagnosis of incipient proximal caries: an ex-vivo study. Braz Dent J. 2008;19:97-102.
- Marshall K, Berry TG, Woolum J. Tooth whitening: current status. Compend Contin Educ Dent. 2010;31:486-492, 494-495.
- Hewlett ER. Etiology and management of whitening-induced tooth hypersensitivity. J Calif Dent Assoc. 2007;35:499-506.
From Dimensions of Dental Hygiene. September 2011 Supplement; 9(9): 54-57.



