
Surface Disinfection Strategies
The following protocols will help ensure effective asepsis for clinical surfaces and equipment.
The dental profession relies heavily on disinfectants to manage clinical contact surfaces (those that do not come into direct contact with patients’ oral tissues) that may become contaminated with bodily fluids during dental treatment. Contamination may occur from contact with the oral health professionals’ gloved hands, or through contact with spray, spatter, or droplets containing a patient’s oral fluids. As technology has advanced, oral health professionals have also come to rely on impervious barriers for management of sensitive semi-critical equipment. The proper use and application of disinfectants and barriers are essential to ensuring patient safety, and preventing occupational injury or illness due to unsafe exposure.

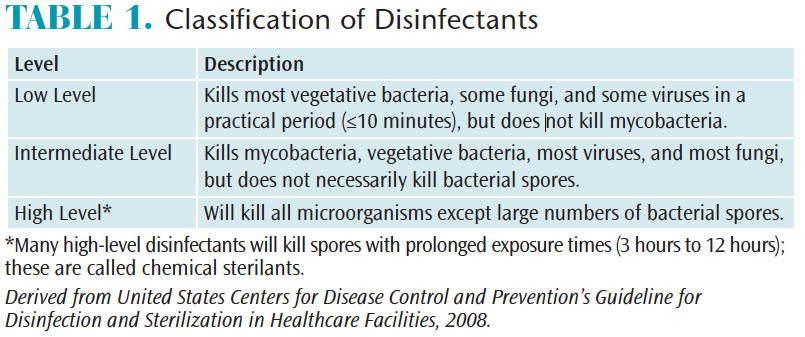
Disinfectants may be divided into three broad categories; low-level, intermediate-level, and high-level disinfectants (Table 1).1 All of these disinfectants have claims for killing human immunodeficiency virus and hepatitis B virus, but only intermediate-level disinfectants can claim to inactivate Mycobacterium tuberculosis. Low-level disinfectants are appropriate for contaminated surfaces that do not have visible blood.2Intermediate-level disinfectants with a tuberculocidal claim should be used on surfaces visibly contaminated with blood.2 Some high-level disinfectants are capable of eliminating all microorganisms under specific circumstances, and are therefore considered sterilants.1 The use of liquid chemical sterilants usually requires a prolonged contact time of at least 20 minutes and up to several hours.1 High-level disinfectants or liquid chemical sterilants are intended for immersion of heat sensitive items that have contacted oral tissues and should never be used for environmental or clinical contact surfaces.
In the United States, disinfectants are regulated by two government agencies. The US Environmental Protection Agency (EPA) regulates antimicrobial products as pesticides.3 Manufacturers of disinfectants must demonstrate that their products are effective against the organisms listed on the product label.3 The EPA also tests products that have been submitted for approval as hospital disinfectants and provides a list of products the agency has tested.4
Disinfectants that make a claim for high-level disinfection or sterilization must be registered with the US Food and Drug Administration (FDA).5 These products are used for immersion of critical and semi-critical devices or instruments that cannot be heat sterilized. The active ingredients in these solutions include glutaraldehyde, ortho-phthaldehyde, hydrogen peroxide, peracetic acid, and hypochlorous acid/hypochlorite.
DISINFECTION
The use of liquid chemical disinfectants should be limited to clinical contact surfaces, and devices or equipment that cannot be heat sterilized.2 Dental devices, instruments, and equipment that can be detached from power sources and are heat stable should be heat sterilized.2 Heat stable or disposable critical or semi-critical instruments and devices should be used when possible. Disinfection is an inferior process to sterilization for several reasons, including the inability to monitor the success of the chemical process, lack of packaging for instruments and devices, and hazards associated with chemical products. The disinfection process may be compromised by both human error and the limitations of disinfectant products. Some of the issues that may result include the level of prior cleaning of the object or surface, presence of organic or inorganic debris, type and level of microbial contamination, concentration of the germicide, exposure time, presence of biofilm, and the temperature and pH of the disinfectant.2 The need to use high-level disinfectants and liquid chemical sterilants in dentistry should be limited because most critical and semi-critical dental instruments and devices are available in either heat stable or disposable versions.1
Following manufacturer instructions for use of disinfectant products is critical to effective asepsis. The need to preclean surfaces, contact time for all microorganisms to be inactivated, storage, shelf life, and use of personal protective equipment (PPE) should be reviewed before using a product. All oral health professionals should receive training in the proper use of disinfectants.6 Surfaces that may contain debris, such as blood, saliva, or dental materials, should be precleaned prior to disinfection. A disinfectant should not be used for precleaning surfaces unless the manufacturer has indicated the product is suitable.7 While some disinfectants are also cleaners, it may be necessary to use a separate product for precleaning surfaces.
 Surface disinfectants are available as liquid sprays or premoistened towelettes. Once applied, the surface should remain wet with the disinfectant for the contact time indicated on the label.8 Generally, either a spray-wipe-spray technique or wipe-discard-wipe technique should be used. When using disinfectant sprays, oral health professionals should first spray the surface and wipe to clean, followed by application of the spray disinfectant. When using premoistened wipes, clinicians should use wipes to clean surfaces, then discard the wipes and use fresh wipes to apply the disinfectant.
Surface disinfectants are available as liquid sprays or premoistened towelettes. Once applied, the surface should remain wet with the disinfectant for the contact time indicated on the label.8 Generally, either a spray-wipe-spray technique or wipe-discard-wipe technique should be used. When using disinfectant sprays, oral health professionals should first spray the surface and wipe to clean, followed by application of the spray disinfectant. When using premoistened wipes, clinicians should use wipes to clean surfaces, then discard the wipes and use fresh wipes to apply the disinfectant.
The antimicrobial effectiveness of some disinfectant products may be affected by contact with certain fabrics used with spray disinfectants. For example, quaternary ammonium compounds interact with cotton and microfiber cloths, resulting in reduced concentrations of the active ingredient delivered to the surface.8 When using spray disinfectants, the manufacturer’s instructions should be consulted for limitations on the types of wipes that may be used with that product.
Most disinfectants are intended for use on hard, nonporous surfaces, and are not suitable for managing contamination of areas, such as upholstered chairs in dental waiting rooms. Some products also stain or discolor surfaces. Recently, approved products containing 30% ethanol have a label claim to kill bacteria and viruses on soft surfaces, such as upholstery, in a single application. They may also be used on hard surfaces. These products do not require precleaning of surfaces and are applied as a spray. Previous ethanol-based disinfectants have had a higher concentration of ethanol, which, in some cases, is less effective in killing microorganisms—and also raises concerns regarding flammability and evaporation.9
IMPERVIOUS BARRIERS
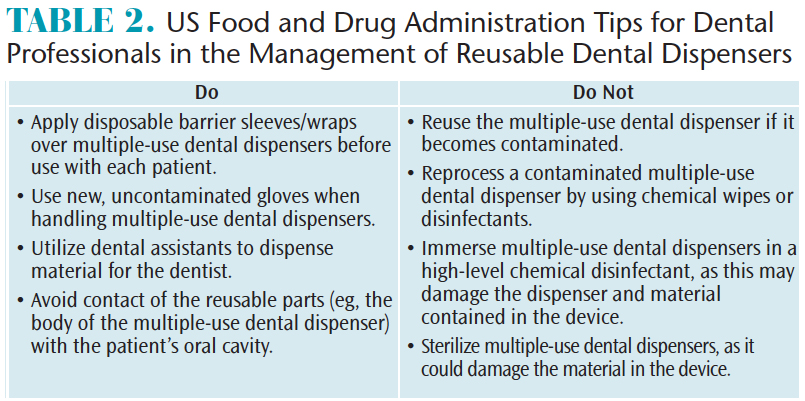
Impervious barriers may be used to protect clinical contact surfaces and semi-critical devices that cannot be heat sterilized, such as digital X-ray sensors, heat sensitive components of cordless dental hygiene handpieces, and multiple-use dispensers for etchants and sealants.2 Multiple-use dispensers usually have disposable tips, but the dispenser itself should be heat sterilized or high-level disinfected between uses. The FDA issued an alert advising that dispensers that have become contaminated with bodily fluids due to contact with the patient’s lips or mucosa should be discarded.10 The FDA provides a number of strategies to reduce the risk of cross-contamination between patients when using multiple-use dental dispensers (Table 2).
Barrier products are cleared by the FDA as medical devices and should have specific instructions for use from the manufacturer. The barrier should be adequate to cover the entire surface, and must be changed between patients. If it is possible that the underlying surface has become contaminated because the barrier has been compromised or the surface was touched with contaminated gloves, it should be cleaned and disinfected before a new barrier is placed.
 The integrity of impervious barriers may be compromised by how they are handled, length of time they remain on surfaces or equipment, and barrier material and thickness.11 A study12 demonstrated that, even under carefully controlled circumstances, digital sensors often became contaminated during extraction from barrier envelopes. In addition, several studies have shown that contamination of barrier-encased digital sensors occurred either through perforation of barriers or for unexplained reasons.11–13 As barriers may provide incomplete protection, the application of a liquid disinfectant between patients has been recommended; this is in addition to a new barrier for each patient. One study also found that using a double barrier reduced the likelihood of contamination of barrier-protected sensors.11
The integrity of impervious barriers may be compromised by how they are handled, length of time they remain on surfaces or equipment, and barrier material and thickness.11 A study12 demonstrated that, even under carefully controlled circumstances, digital sensors often became contaminated during extraction from barrier envelopes. In addition, several studies have shown that contamination of barrier-encased digital sensors occurred either through perforation of barriers or for unexplained reasons.11–13 As barriers may provide incomplete protection, the application of a liquid disinfectant between patients has been recommended; this is in addition to a new barrier for each patient. One study also found that using a double barrier reduced the likelihood of contamination of barrier-protected sensors.11
Cordless dental hygiene handpieces are semi-critical devices with certain components that cannot be heat sterilized. Existing recommendations state that all devices, such as handpieces attached to an air supply or waterline, should be heat sterilized between uses.2 Even though battery-driven handpieces are not attached to air lines, they are still semi-critical devices and are preferably reprocessed using heat sterilization methods. Some manufacturers have designed the cordless hygiene handpiece with autoclavable outer sheaths and handpiece holders. This provides a mechanism for sterilizing the parts that may come into contact with oral fluids or patient tissues, while protecting the heat-sensitive battery.
OCCUPATIONAL SAFETY
With the widespread use of disinfectants in the dental setting, it may be easy to overlook that these chemical germicides may carry risks if improperly applied. The use of some high-level disinfectants has been associated with skin irritation and sensitization, as well as respiratory problems, such as dermatitis and asthma.14–18 Glutaraldehyde has long been known to present a risk for dermatitis and asthma in workers exposed to the vapors in hospital settings.14 Little information exists regarding occupational risks associated with the use of newer high-level disinfectants, although limited reports have implicated other high-level-disinfectant active ingredients to asthma and skin irritation.19,20
The Occupational Safety and Health Administration has released guidelines on the use of glutaraldehyde in health care settings.21Some of the recommendations include the use of local exhaust or ductless exhaust hood containment of glutaraldehyde. A local exhaust hood removes vapors, which pass through a system of ducts to be exhausted outside. Since this is impractical in many dental settings, a ductless fume hood may be used. Ductless fume hoods collect vapors, which pass through a charcoal filter to cleanse the air, which is then returned to the room. Additionally, it is recommended that rooms in which glutaraldehyde is being use have rates of at least 10 air exchanges per hour—an unlikely high number of air exchanges for a typical dental office.
In addition to control of vapors, it is important for oral health professionals to avoid bare-handed contact with high-level disinfectant/sterilants. Consequently, PPE—including fluid-resistant gowns, eye and face protection, and protective gloves—should be worn when handling high-level disinfectants. It may be necessary to wear chemical-resistant gloves, such as those made of nitrile, when handling some chemical germicides. Each manufacturer’s instructions for use should be reviewed regarding which specific PPE is appropriate.6
High-level disinfectants should be thoroughly rinsed from the surface of equipment or devices to avoid irritation of the patient’s mucosa. One of the problematic areas of using high-level disinfectants as a sterilant is the inability to package the item prior to processing. This prevents clinicians from storing such items in a sterile pack. Limiting the use of high-level disinfectants by selecting devices that are either heat-stable (ie, can be sterilized) or disposable, single-use items is recommended to reduce reliance on liquid chemical disinfectant/sterilants.2 There may be some devices for which no other option is available, however—in which case appropriate precautions should be consistently followed and the least hazardous product selected.
Before a chemical product is used, oral health professionals must review the information found on the safety data sheet (SDS) included with the product.6 If the manufacturer does not provide an SDS with the product, it should be requested and the product should not be used until the SDS is reviewed. It will contain important information for safe use and any risks associated with the product. The manufacturer’s label will contain additional information, such as the active ingredient, proper application, storage, use, and efficacy of the product.
Liquid chemical disinfectants and impervious barriers are important tools for infection prevention in dental settings—however, they have limitations that should be considered when making decisions regarding clinical asepsis. Manufacturers of reusable products and devices should provide information regarding how to safely reprocess items. If a manufacturer cannot provide written instructions, the product should not be used. As dental devices become increasingly complex, manufacturers must provide adequate reprocessing instructions, and clinicians must follow instructions diligently. At times, this will require multiple products or processes in order to safely and effectively manage the devices and surfaces to ensure safe care.
REFERENCES
- Centers for Disease Control and Prevention. Guideline for Disinfection and Sterilization in Healthcare Facilities, 2008. Available at: cdc.gov/infectioncontrol/pdf/guidelines/disinfection-guidelines.pdf. Accessed December 22, 2017.
- Centers for Disease Control and Prevention. Guidelines for infection control in dental health-care settings—2003. MMWR. 2003;52:1–61.
- US Environmental Protection Agency. Pesticide Registration. Selected EPA-Registered Disinfectants. Available at: epa.gov/pesticide-registration/selected-epa-registered-disinfectants. Accessed December 22, 2017.
- US Environmental Protection Agency. The Antimicrobial Testing Program—Hospital Disinfectant and Tuberculocidal Products Tested or Pending Testing. Available at: epa.gov/pesticide-registration/hospital-disinfectant-and-tuberculocidal-products-tested-or-pending-testing. Accessed December 22, 2017.
- US Food and Drug Administration. FDA Guidance Information. FDA-Cleared Sterilants and High Level Disinfectants. Available at: fdaguidance.net/2014/04/fda-cleared-sterilants-and-high-level-disinfectants/. Accessed December 22, 2017.
- Occupational Safety and Health Administration. Hazard Communication Standard. CFR 1910.1200. Federal Register. March 26, 2012.
- Centers for Disease Control and Prevention. Summary of Infection Prevention Practices in Dental Settings: Basic Expectations for Safe Care. Available at: cdc.gov/oralhealth/infectioncontrol/pdf/safe-care.pdf. Accessed December 22, 2017.
- Rutala WA, Weber DJ. Monitoring and improving the effectiveness of surface cleaning and disinfection. Am J Infect Cont. 2016;44:e69–e76.
- Alhmidi H, Koganti S, Cadnum JL, Rai H, Lenscon AL, Donskey CJ. Evaluation of a novel alcohol-based surface disinfectant for disinfection of hard and soft surfaces in healthcare facilities. Open Forum Infect Dis. 2017;25:4.
- US Food and Drug Administration. Multiple-Use Dental Dispenser Devices. Available at: fda.gov/MedicalDevices/ProductsandMedicalProcedures/DentalProducts/ucm404472.htm. Accessed December 22, 2017.
- Choi JW. Perforation rate of intraoral barriers for direct digital radiography. Dentomaxillo Radiol. 2015;44:20140245.
- MacDonald DS, Waterfield JD. Infection control in digital intraoral radiography: evaluation of microbiological contamination of photostimulable phosphor plates in barrier envelopes. J Can Dent Assoc. 2011;77:93.
- Hokett SD, Honey JR, Ruiz F, Baisden MK, Hoen MM. Assessing the effectiveness of direct digital radiography barrier sheaths and finger cots. J Am Dent Assoc. 2000;131: 463–467.
- Henn SA, Boiano JM, Steege AL. Precautionary practices of healthcare workers who disinfect medical and dental devices using high-level disinfectants. Infect Control Hosp Epidemiol. 2015;36:180–185.
- Centers for Disease Control and Prevention. Glutaraldehyde—Occupational Hazards in Hospitals. Available at: cdc.gov/niosh/docs/2001-115/default.html. Accessed December 22, 2017.
- Fowler JF Jr. Allergic contact dermatitis from glutaraldehyde exposure. J Occup Med. 1989;31:852–853.
- Nethercott JR, Holness DL, Page E. Occupational contact dermatitis due to glutaraldehyde in health care workers. Contact Dermatitis. 1988;18:193–196.
- Waters A, Beach J, Abramson M. Symptoms and lung function in health care personnel exposed to glutaraldehyde. Am J Ind Med. 2003;43:196–203.
- Fujita H, Ogawa M, Endo Y. A case of occupational bronchial asthma and contact dermatitis caused by ortho-phthalaldehyde exposure in a medical worker. J Occup Health. 2006;48:413–416.
- Cristofari-Marquand E, Kacel M, Milhe F, Magnan A, Lehucher-Michel MP. Asthma caused by peracetic acid-hydrogen peroxide mixture. J Occup Health. 2007;49:155–158.
- Occupational Safety and Health Administration. Best Practices for the Safe Use of Glutarladehyde in Health Care. Available at: osha.gov/Publications/glutaraldehyde.pdf. Accessed December 22, 2017.
From Dimensions of Dental Hygiene. January 2018;16(01):24,26-28.

