 JOSE LUIS CALVO MARTIN & JOSE ENRIQUE GARCIA-MAURIÑO MUZQUIZ/ISTOCK/GETTY IMAGES PLUS
JOSE LUIS CALVO MARTIN & JOSE ENRIQUE GARCIA-MAURIÑO MUZQUIZ/ISTOCK/GETTY IMAGES PLUS
Strategies for Addressing Sialorrhea
This salivary gland dysfunction presents a multidimensional challenge to oral health professionals who must be well versed in sialorrhea’s causes and progression in order to effectively treatment plan.
This course was published in the April 2019 issue and expires April 2022. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the anatomy and function of the salivary glands.
- Define sialorrhea.
- Identify the diagnosis and treatment of sialorrhea and adverse effects of such treatments.
- Explain the oral health considerations of sialorrhea and its treatment.
Thanks to its complex biochemical composition and physical properties, saliva is an essential player in a variety of physiological processes, including taste and smell sensation, digestion, food bolus formation, mastication and swallowing, and articulation of speech. It provides protection of oral tissues by lubricating and forming a mucin layer, buffering the effects of food and drink with variations of temperature and pH. Additionally, antimicrobial effects of the many salivary proteins and peptides maintain the balance of oral microflora and keep the pathogenic microorganisms at sufficiently low levels to prevent oral disease.1,2
As saliva is hypersaturated with calcium, phosphate, and fluoride ions, and able to form acquired enamel pellicle, a protective protein, within seconds on enamel surfaces, it provides anti-cariogenic effects and protects against enamel loss.1 The disfunction of salivary production and clearance can have significant consequences contributing to oral and systemic disease. While much attention has been appropriately given to salivary hypofunction, or xerostomia, sialorrhea—often referred to as drooling—is another disorder of salivary function. It can occur due to actual increase in saliva production or, more frequently, accompany neurological conditions that affect salivary clearance.
ANATOMY, FUNCTION, AND NEURAL REGULATION
Three pairs of major salivary glands (parotid, submandibular (Figure 1), and sublingual) and up to 1,000 minor salivary glands, located in the labial, buccal, palatal, lingual and retromolar oral mucosa, make up the total daily salivary volume of about 0.6 L.1,2 While most of the saliva is produced by the major glands (90%), the importance of the minor glands, which are nameless except for the von Ebner’s located in the circumvallate papillae of the tongue, should not be overlooked. The minor glands provide a relatively large portion of the salivary mucins needed for lubrication.2 Salivary output varies during the day in response to food intake and circadian rhythm, with peak flow in the late afternoon and lowest secretion during the night (as low as 5% of daytime secretion).1,3 Contributions of each of the major salivary glands during mastication (stimulated saliva) and rest (unstimulated saliva) are different, as well as the composition of their secretions. Watery, low in protein but high in amylase saliva, mostly produced by the parotid glands, is necessary during mastication, food bolus formation, early digestion, and swallowing. In contrast, protein- and mucin-rich unstimulated saliva, secreted continuously at about 0.3 ml to 0.4 ml per minute, is mostly produced by the submandibular glands and serves as a lubricant. Continuous unstimulated flow prevents retrograde infection of the salivary glands by the oral bacteria.1
Autonomic nervous regulation of the salivary glands is provided by both parasympathetic (via facial and glossopharyngeal cranial nerves) and sympathetic divisions (via sympathetic trunk/superior cervical ganglion) that interact synergistically.4 Parasympathetic innervation is more abundant and produces large volumes of watery saliva. Sympathetic stimulation results in production of small volumes of thicker, protein-rich saliva.2–4
Blood supply to the glands is influenced by the parasympathetic input dilating the blood vessels and providing the water necessary for saliva production; during food intake it is increased up to 20-fold. Parasympathetic innervation impacts the gland’s size and secretory capacity. Denervation results in temporary and reversible weight and salivary output reduction.2 This is important for understanding the mechanism of some of the sialorrhea treatment approaches that include surgical gland denervation and injections of botulinum neurotoxins (BoNTs) that temporarily disrupt parasympathetic stimulation and therefore reduce salivary output.
SIALORRHEA DEFINED
Sialorrhea, also known as ptyalism, is a disorder of excessive saliva production or inadequate clearance of normal amounts of saliva from the oral cavity.3–5 Ranging from mild to severe, it can manifest as saliva pooling in anterior oral cavity or anterior, over the lip, drooling or posterior pharyngeal flow.6,7 Actual hyperproduction of saliva is rare and can be idiopathic or drug-induced. Most frequently, sialorrhea is a symptom of neurological disorders associated with neuromuscular or sensory dysfunction that leads to impaired control and coordination of the orofacial, lingual, pharyngeal and soft palate muscles. This affects swallowing, a complex multiphased process of oral cavity clearance.3,6,7
submandibular gland.
Drooling is common in typically developing infants and young children until the development of salivary continence by age 4, after which it is considered abnormal.6,7 In children, sialorrhea is most commonly associated with cerebral palsy (CP), affecting as many as 10% to 58% of patients. It can also accompany intellectual and developmental disabilities.6–9 In adults, the most common association is with Parkinson’s disease (PD) with a prevalence of 70% to 80%.5,7 Other, less common causes include amyotrophic lateral sclerosis (ALS), pseudobulbar palsy, and stroke.5,7,9 Sialorrhea may be caused by medication use, especially clozapine, an antipsychotic used to manage schizophrenia.3,7
Contributing factors to sialorrhea include anatomical/orthodontic considerations (anterior open bite, malocclusion, macroglossia); insufficient lip closure; difficulty controlling the lips, tongue, and mandible; decreased intraoral sensitivity; and inadequate head/body posture.4,5,10 Regardless of the cause, sialorrhea presents a number of adverse symptoms, including oral/perioral, local, general, psycho-social, and emotional outcomes, as well as substantial burden on caregivers (Table 1). Due to its complexity, it also presents a management challenge requiring a multidisciplinary approach to improve patients’ symptoms and their quality of life.4,9
DIAGNOSIS, TREATMENT, AND ADVERSE EFFECTS
Objective measurements of saliva production are useful in research, however, they are difficult to carry out in clinical environments. Detailed patient histories and long-term observations of patients and caregivers are more informative.4,9
Because of the varying etiology and sequelae of sialorrhea, treatment interventions depend on presentation, contributing factors, associated medical conditions, outcome expectations, and risk of adverse effects.5,9,11 The goals of treatment include reduction of salivary output, reduction of severity and frequency of drooling, and consideration of patient and caregiver satisfaction.11,12 Treatment options include: conservative behavioral and physical therapies; pharmacologic approaches, including BoNT injections; acupuncture; radiotherapy; and more invasive surgical interventions, such as neurectomy, salivary duct ligation or relocation, and salivary gland excision.5,6,9,11,13 As assessments and interventions are multidimensional, an interprofessional team approach has been suggested. For oral health professionals, this may include caries treatment and prevention and correcting malocclusion as needed prior to specific sialorrhea treatment.4
BEHAVIORAL MODIFICATION, PHYSICAL MODIFICATION, PHYSICAL THERAPIES AND OROSENSORY APPROACHES
Noninvasive approaches, mainly studied on children with CP, focus on saliva clearance by improving oral motor dysfunction and inducing more frequent swallowing. Simple physical therapy exercises emphasize more erect posture and improvement of facial and lip muscle tone through playful exercises to reduce anterior drooling.6,10
Orosensory therapy may include muscle stimulation through vibration with a device, and oral and facial physiotherapy may reduce tongue protrusion, and improve lip closure.4,11 Devices such as the one developed by Castillo-Morales, which uses a palatal plate and buccal and lingual stimulators to induce sucking and tongue retrusion, have shown good results for children older than age 4.4,9,10
Biofeedback uses electromyography to target the muscle groups used in swallowing. Auditory and visual cues inform the patient, eliciting changes in swallowing reflex and voluntary swallowing.4,6 These approaches require early intervention, sometimes begun in infancy, repetition, and time commitment.4,9 Long-term results are not seen in adults with PD,5 but low incidence of adverse events suggests more trials are warranted for these interventions.11
Rehabilitative treatments in adults with PD include an evaluation of swallowing reflex and are aimed at improving food and saliva clearance and reducing aspiration risk.14 Various interventions—such as surface electrical stimulation, video-assisted swallowing with compensatory exercises, thermal tactile stimulation, and gum chewing—have mild to moderate improvements but often lack sustained results.14 Limited research has shown some effectiveness of tongue acupuncture in decreasing drooling measures as an alternative to surgery.11 Radiotherapy of selected portions of the parotid and submandibular glands has been investigated due to its ability to reduce saliva production in adults with PD and ALS.5,10 Recent studies have good results, but this therapy is restricted to older adults and patients with ALS due to the inherent risks associated with radiation exposure.10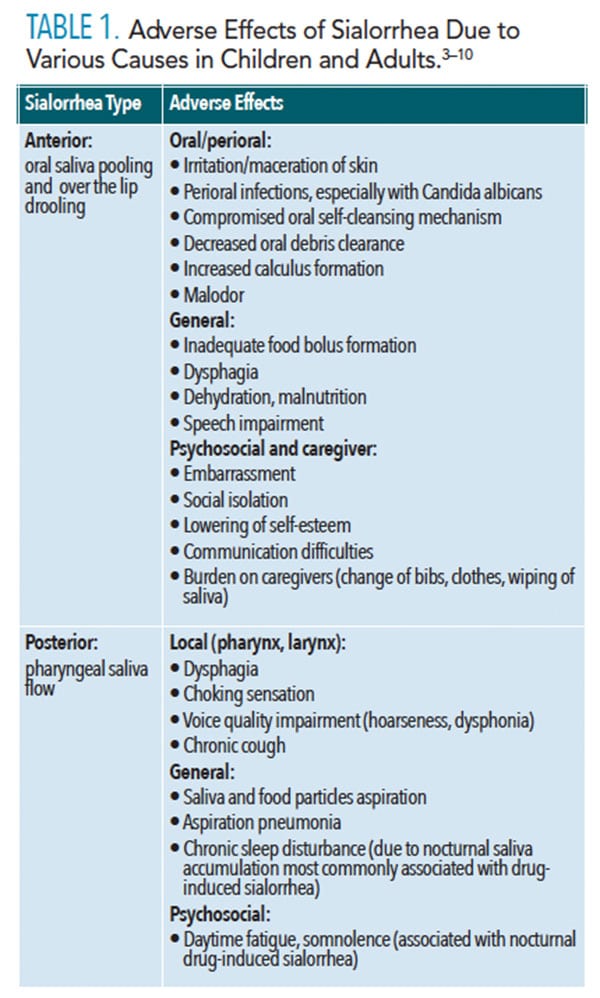
PHARMACOLOGIC APPROACHES
Less invasive strategies to reduce the effects of sialorrhea also include pharmacologic interventions, primarily with anticholinergic drugs, and the use of BoNT injections. Because anticholinergic agents block the release and binding of acetylcholine to the muscarinic cholinergic receptors in the salivary glands, salivary volume is reduced.2 For children with CP and other neurological disorders associated with sialorrhea, and for adults with PD, scopolamine, most often as a transdermal patch affixed behind the ear, has been effective.4
Glycopyrrolate, which is approved by the United States Food and Drug Administration to treat sialorrhea in children with CP, can be administered as an oral solution, and may be preferred as it does not cross the blood-brain barrier.5 Atropine, benztropine, and trihexyphenidyl are also used in adults and children, and all carry risk of anticholinergic side effects, including constipation, urinary retention, photophobia, restlessness, and irritability.11,12 For clozapine-induced sialorrhea, α2-adrenergic receptor agonists, such as clonidine may be effective, as clozapine is an α2-antagonist, and anticholinergics are also prescribed.3,5
BoNT injections effectively reduce salivary output in adults and children, and most adverse effects are mild and transient.5,11,15 BoNT type A or B is delivered locally into either parotid or submandibular glands or both and, although anatomical landmarks may be used, the most precise delivery and highest safety profile is achieved when injections are guided by ultrasound.4,7,15 Local anesthesia may be used, and general anesthesia is more common with children.7 The ability of BoNT to block the release of acetylcholine at the neuroeffector junction in the salivary glands has been shown to decrease secretions and drooling for 3 months to 9 months.16 Injections require repetition, as glandular function is gradually restored upon formation of new neuro-effector connections.2,17
Adverse effects may include transient dysphagia, increased saliva thickness and xerostomia along with associated oral and systemic risks, but research demonstrates that low-dose, ultrasound guided BoNT-A injections into the parotid and submandibular glands of children are safe and effective in decreasing salivary flow rate with no decrease in salivary pH or increased cariogenic bacterial count,15 and are effective and well-tolerated by adults with PD.5 Other considerations are expense and the need for repeated sedation, especially with children.7
Combination therapies may work synergistically to reduce adverse effects of single-treatment therapy. One recent retrospective study investigated the adjunctive use of anticholinergic drugs, primarily glycopyrrolate and scopolamine combined with BoNT injections guided by ultrasonography, as primary treatment of sialorrhea for 112 neurologically impaired children at risk for aspiration and pneumonia over more than 9 years. The purpose was to mediate the drug tolerance and possible tachyphylaxis effects of the anticholinergics, while reducing the frequency of BoNT injections. The results showed fewer pneumonia episodes and hospitalizations, decreased BoNT injection frequency, and more tolerable dosages of the anticholinergic drugs.12
SURGICAL INTERVENTION
Failure of less invasive therapies to reduce sialorrhea and its effects may require a surgical approach, which includes neurectomy, salivary duct ligation or relocation, or salivary gland excision.4,10,12 Neurectomy involves sectioning the tympanic plexus and the chorda tympani nerves to reduce the salivary flow rate of the submandibular and sublingual glands, effectively reducing drooling. Hearing loss and reduction of taste are significant and common risks.10 Ligation of the parotid gland ducts via ligatures or laser produces stenosis of the duct and gland atrophy. Submandibular gland relocation posteriorly may stimulate swallowing reflex but carries risk of aspiration. Adverse events in duct ligation and/or relocation also include transient swelling, ranula, cyst formation, and infection.10 Finally, excision of the submandibular glands, sometimes combined with parotid duct ligation, is effective, but is irreversible, carrying aforementioned risks, plus paralysis and scarring.4,10,12
ORAL/DENTAL CONSIDERATIONS
Regardless of the intervention, the goal of sialorrhea treatment is a reduction in salivary volume, which may affect self-cleansing of the oral cavity and predispose patients with neurological conditions and special needs to increased risk for dental caries and periodontal and mucosal diseases. However, the complex relationship between salivary flow amount/rate, saliva viscosity, pH, and protein composition and caries risk is not well understood.18,19
Studies that evaluated dental caries risk as an adverse effect of sialorrhea treatment focused on the various regimens of BoNT therapies16,17,20,21 Although inconclusive due to small patient samples and the use of various techniques, these investigations underscore the risk of dental disease as a consequence of reduced saliva flow, viscosity, and pH, and stress the importance of balancing the need for effective control of sialorrhea with maintaining oral health.
To that end, these studies emphasize the need for comprehensive oral evaluation and self-care education prior to sialorrhea treatment, followed by regular check-ups and interventions.16,17,21 In a striking example, children treated with BoNT injections were 1.73 times more likely to develop decay vs a placebo,20 in the absence of any special dental hygiene care or instructions. On the other hand, no new caries lesions developed in the study that included pre-treatment evaluation and oral hygiene instructions.16 This highlights the need for comprehensive dental hygiene care using preventive strategies such as fluoride varnish applications and patient/caregiver education.
A thorough evaluation of the major and minor salivary glands and their function is an important component of extra- and intraoral examination. Coupled with a detailed medical and dental history and medication use, it provides key information for identifying salivary gland disease and dysfunction that can compromise oral health, and offers the basis for professional and self-care interventions. Multidisciplinary collaboration including dentists, dental hygienists, primary care providers, neurologists, oral surgeons, and other professionals is essential for successful treatment outcomes.
REFERENCES
- Dawes C, Pedersen AML, Villa A, et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch Oral Biol. 2015;60:863–874.
- Pedersen AML, Sørensen CE, Proctor GB, Carpenter GH, Ekström J. Salivary secretion in health and disease. J Oral Rehabil. 2018;45:730–746.
- PraharaJ SK, Arora M, Gandotra S. Clozapine-induced sialorrhea: pathophysiology and management strategies. Psychopharmacology (Berl). 2006;185:265–273.
- Iro H, Zenk J. Salivary gland diseases in children. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2014;13:Doc06.
- Chou KL, Evatt M, Hinson V, Kompoliti K. Sialorrhea in Parkinson’s disease: a review. Mov Disord. 2007;22:2306–2313.
- Scofano Dias BL, Fernandes AR, Maia Filho H de S. Sialorrhea in children with cerebral palsy. J Pediatr Versão Em Port. 2016;92:549–558.
- LakraJ AA, Moghimi N, Jabbari B. Sialorrhea: anatomy, pathophysiology and treatment with emphasis on the role of botulinum toxins. Toxins. 2013;5:1010–1031.
- Hegde A, Shetty YR, Pani SC. Drooling of saliva and its effect on the oral health status of children with cerebral palsy. J Clin Pediatr Dent. 2008;3:235–238.
- Hockstein NG, Samadi DS, Gendron K, Handler SD. Sialorrhea: a management challenge. Am Fam Physician. 2004;69:2628–2634.
- Meningaud JP, Pitak-Arnnop P, Chikhani L, Bertrand J-C. Drooling of saliva: a review of the etiology and management options. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontology. 2006;101:48–57.
- Walshe M, Smith M, Pennington L. Interventions for drooling in children with cerebral palsy. Cochrane Database Syst Rev. 2012;2:CD008624.
- Dohar JE. Sialorrhea and aspiration control—a minimally invasive strategy uncomplicated by anticholinergic drug tolerance or tachyphylaxis. Int J Pediatr Otorhinolaryngol. 2019;116:97–101.
- Ye Q, Xie Y, Shi J, Xu Z, Ou A, Xu N. Systematic review on acupuncture for treatment of dysphagia after stroke. Evid-Based Complement Altern Med ECAM. 2017;2017:1–18.
- van Hooren MRA, Baijens LWJ, Voskuilen S, Oosterloo M, Kremer B. Treatment effects for dysphagia in Parkinson’s disease: a systematic review. Parkinsonism Relat Disord. 2014;20:800–807.
- Wu KPH, Ke JY, Chen CY, Chen CL, Chou MY, Pei YC. Botulinum toxin type a on oral health in treating sialorrhea in children with cerebral palsy: a randomized, double-blind, placebo-controlled study. J Child Neurol. 2011;26:838–843.
- Møller E, Pedersen SA, Vinicoff PG, et al. Onabotulinumtoxin A treatment of drooling in children with cerebral palsy: a prospective, longitudinal open-label study. Toxins. 2015;7:2481–2493.
- Møller E, Karlsborg M, Bardow A, Lykkeaa J, Nissen FH, Bakke M. Treatment of severe drooling with botulinum toxin in amyotrophic lateral sclerosis and Parkinson’s disease: efficacy and possible mechanisms. Acta Odontol Scand. 2011;69:151–157.
- Hemadi AS, Huang R, Zhou Y, Zou J. Salivary proteins and microbiota as biomarkers for early childhood caries risk assessment. Int J Oral Sci. 2017;9:e1.
- Bhalla S, Tandon S, Satyamoorthy K. Salivary proteins and early childhood caries: a gel electrophoretic analysis. Contemp Clin Dent. 2010;1:17–22.
- Dos Santos BF, Dabbagh B, Daniel SJ, Schwartz S. Association of onabotulinum toxin A treatment with salivary pH and dental caries of neurologically impaired children with sialorrhea. Int J Paediatr Dent. 2016;26:45–51.
- Tiigimäe-Saar J, Taba P, Tamme T. Does Botulinum neurotoxin type A treatment for sialorrhea change oral health? Clin Oral Investig. 2017;21:795
From Dimensions of Dental Hygiene. May 2019;17(5):34–36,39.




Thank you for the thoughtful article. This is not a topic I read a lot about. But this was well presented and will help me communicate with my patients/caregivers in a thoughtful and scientific way.