
Stain Removal With Polishing
Oral health professionals need to be knowledgeable about the causes behind staining, the correlation between oral pH and dental erosion, and appropriate polishing choices for different patient types.
The polishing of natural teeth and restorations is integral to the standard prophylaxis performed by dental hygienists. While the factors involved in stain accumulation have changed, patients still desire the clean, smooth feeling provided by polishing at the end of the dental hygiene appointment. Dental hygienists should be well versed in the causes behind staining, the correlation between oral pH and dental erosion, and appropriate polishing choices for different patient types. As the typical American lifestyle has undergone much change over the past few decades, so has the status of tooth discoloration. Dietary and social habits greatly impact dental stain accumulation. Smoking tobacco/marijuana and chewing or dipping tobacco obviously affect extrinsic stain formation, but chemical agents may also play a role in the complexity of stain buildup. The use of stannous fluoride, antibiotics, and chlorhexidine, as well as exposure to iron in drinking water impact the color and intensity of extrinsic stain.1 Dietary changes include an increase in the amount and frequency of consumption of stain-producing and acidic beverages, such as coffee, tea, wine, and sports drinks.
Wine and other alcoholic beverages can lower the pH of the mouth, increasing the risk of erosion.2,3 Consuming acidic beverages, such as wine, soda pop, fruit juices, and sports/energy drinks, contribute to enamel demineralization and erosion by decreasing the pH levels of the mouth. When the tooth surface pH falls below 5.5, these areas become more susceptible to deterioration.4 The properties of these drinks can create a chemical degradation of esthetic restorative materials, resulting in increased plaque and stain retention.5
Patients can control these environmental factors through dietary choices. Reducing the consumption of acidic foods/beverages and minimizing sugar intake can help to balance the oral ecosystem. Maintaining a healthy salivary flow provides a natural cleansing and buffering environment, in which remineralization can occur when calcium ions bond with saliva.6
ADDRESSING STAIN
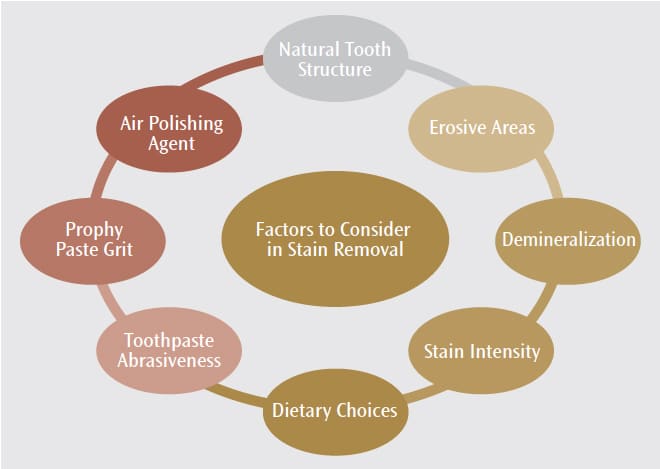
Several factors need to be evaluated when determining the treatment for stained teeth and restorations (Figure 1). According to Spina et al,7 some composite materials are more susceptible to stain. The researchers looked at the discoloration effects of water (control), cola-based soft drink, coffee, and wine on resin composite materials. The results indicated that the color intensity on the composite restorations was not significantly affected by cola-based drinks, but it was negatively impacted by coffee and wine. Results also showed that microfilled and nanohybrid restorations that used small filler-size particles may be more resistant to staining and that the bonding structure of specific restorative materials may contribute to surface irregularities, which can increase stain retention. The pH levels of the drinks used in this study may have also contributed to the color degradation results. In the end, the authors concluded that color stability is related to the type of restorative material used and that the types of drinks consumed by patients also impact restorative materials’ color stability.7
Abrasive ingredients in toothpastes include silica, calcium carbonate, perlite, and sodium bicarbonate. These components offer varying degrees of abrasiveness to cleanse the teeth and remove stain. The abrasive action of toothpaste removes superficial stain on dental surfaces but does not change the color of the teeth. These ingredients used in both regular and whitening toothpastes reduce stain by physically thinning the stain layers with an abrasive action.8 In an in vitro study, Choi et al9 used scanning electron microscopy and atomic force microscopy to look at the effects of the amount of brushing (5, 15, and 30 minutes) and the timing of brushing after the consumption of a cola-based soft drink (0, 30, 60, and 120 minutes after consumption) on dentin loss. Their results showed that patients should refrain from toothbrushing for at least 60 minutes after consuming acidic beverages to reduce abrasion and erosion to tooth surfaces. However, the duration of brushing had no effect.
The International Standard Organization (ISO) asserts that the abrasiveness of whitening toothpastes should not exceed the relative dentine abrasivity (RDA) score of 250. Whitening toothpastes vary in range from medium (RDA: 60 to 100) to high (RDA: 100) abrasivity.10 Tooth whitening from abrasive toothpastes can generally be accomplished within 4 weeks. According to Patil et al,10 the use of abrasive whitening toothpastes beyond 4 weeks will not improve the whiteness of teeth. Some studies show the efficacy of the addition of chemical components in toothpastes, such as sodium phytate and sodium tripolyphosphate.11–14 The use of enzymatic whitening toothpastes may produce less traumatic results with equal stain removal as more abrasive options.10
PROPHYLAXIS
Several factors should be considered when selecting a rubber cup polishing agent for stain removal. The hardness, particle shape, and size affect the abrasiveness of the polishing agent. The hardness of the polishing agent must be harder than the surface that is being polished. The abrasiveness of an agent is partially determined by the particle shape. An angular particle shape has a sharper edge than spherical particles and will scratch the surface to be polished more aggressively. A smaller particle size will be less abrasive than a larger particle agent. These parameters are distinguishable by the grit description for prophy paste (eg, fine, medium, coarse, or extra coarse). In areas of erosion or demineralization, the clinician should evaluate the use of abrasives on softened tooth structures and determine the value of alternative strategies, such as the use of cleansing-only pastes.15
AIR POLISHING
When using air polishing, the product’s recommended use should be reviewed. Sodium bicarbonate, aluminum hydroxide, calcium carbonate, and bioactive glass may be used for supragingival polishing; however, each varies in particle size, shape, and hardness. Sodium bicarbonate exhibits a 2.5 Mohs scale of hardness, whereas bioactive glass is a 6. These variations will impact the surfaces that can be effectively polished without causing damage to the tooth or restorative surface. Bioactive glass particles have a high Mohs scale of hardness and the abrasive effects on tooth structures and restorations are unknown. Sodium bicarbonate and aluminum hydroxide powders are effective for stain removal on tooth surfaces but are too abrasive for esthetic restorations and dentin/cementum. Metal restorations may be dulled with these abrasive products and utilizing alternative polishing options may be best.16
In addition to the physical properties of air polishing agents, optimum application is essential to minimizing the risk of structural damage. Trauma to tooth structures, restorations, and gingival tissues can occur with improper technique, causing pitting of restorations, loss of marginal integrity, and surface roughness. Technique also influences the abrasive penetration of an agent. The device nozzle design, water settings, and proximity to the treated surface can help control the amount of abrasion to the tooth or restoration. The nozzle spraying distance should be 2 mm to 7 mm from the polishing tip to the tooth surface. This will reduce the risk of damage to existing dental structures.17
The use of low abrasive glycine powder in air polishing has broadened the scope of this technology in dentistry. This technique uses specially designed armamentarium to deliver subgingival irrigation to remove plaque and debris.17 Dental hygienists may consider the benefits of augmenting polishing procedures with subgingival air polishing irrigation to offer stain removal benefits along with biofilm removal benefits.16
CONCLUSION
Many factors should be considered when treating dental stain. The type of stain and affected surface will impact the appropriate mode of treatment. In today’s society, white teeth are highly valued and many patients will present to the dental office desiring a brighter, whiter smile. Oral health professionals are charged with not only providing positive esthetic results and advising patients on how to reduce staining, but helping patients achieve positive oral health, as well.
REFERENCES
- Wilkins E. Clinical Practice of the Dental Hygienist. 11th ed. Philadelphia: Lippincott Williams; 2013:306–309.
- Nutrition Assistance Program Report Series: the Office of Analysis, Nutrition and Evaluation. Children’s Diets in the Mid-1990s: Dietary Intake and its Relationship With School Meal Participation. Available at: https://fns-prod.azureedge.net/sites/default/files/ChilDiet.pdf. Accessed February 21, 2018.
- Wang X, Lussi A. Functional foods/ingredients on dental erosion. Eur J Nutr. 2012;51(Suppl 2):S39–548.
- Dahm T. Skip the sugary drinks. Dimensions of Dental Hygiene. 2015;13:52–57.
- Andreea B, Cristina M, Melinda S. The behavior of composites, glass ionomers and compomers in erosive conditions—in vitro study. Acta Medica Marisiensis. 2014;60:200–203.
- Yoon S. Supporting remineralization. Dimensions of Dental Hygiene. 2015;13:38–42.
- Spina D, Grossi J, Cunali R, et al. Evaluation of discoloration removal by polishing resin composites submitted to staining in different drink solutions. Int Sch Res Notices. 2015;2015:853975.
- Moran J, Claydon N, Addy M, Newcombe R. Clinical studies to determine the effectiveness of a whitening toothpaste at reducing stain (using a forced stain model). Int J Dent Hyg. 2005;3:25–30.
- Choi S, Park KH, Cheong Y, Moon SW, Park YG, Park HK. Potential effects of tooth-brushing on human dentin wear following exposure to acidic soft drinks. J Microsc. 2012;247:176–185.
- Patil P, Ankola A, Hebbal M, Patil A. Comparison of effectiveness of abrasive and enzymatic action of whitening toothpastes in removal of extrinsic stains-a clinical trial. Int J Dent Hyg. 2015;13:25–29.
- Milleman KR, Creeth JE, Burnett GR, Milleman JL. A randomized clinical trial to evaluate the stain removal efficacy of a sodium phytate dentifrice formulation. J Esthet Restor Dent. February 7, 2018. Epub ahead of print.
- Young S, Mason S, Milleman JL, Milleman KR. A randomized clinical study to evaluate the effect of an ultra-low abrasivity dentifrice on extrinsic dental stain. Am J Dent. 2017;30:255–261.
- Hara AT, Turssi CP. Baking soda as an abrasive in toothpastes: Mechanism of action and safety and effectiveness considerations. J Am Dent Assoc. 2017;148(11S):S27-S33.
- Joiner A. Whitening toothpastes: a review of the literature. J Dent. 2010;38(Suppl 2):e17–24.
- Jefferies SR. Abrasive finishing and polishing in restorative dentistry: A state-of-the-art review. Dent Clin N Am. 2007;51:379–397.
- Pence S. The evolution of air polishing. Dimensions of Dental Hygiene. 2015, 13:58–61.
- Graumann SJ, Sensat ML, Stoltenberg JL. Air polishing: a review of current literature. J Dent Hyg. 2013;87:173–180.
From Dimensions of Dental Hygiene. March 2018;16(3):24-26.

