Sensitivity Assistance
Help your patients find relief from dentinal hypersensitivity with these current treatment options.
Dentinal hypersensitivity, often referred to as root sensitivity, is estimated to affect between 8% and 35% of the United States population.1 Many patients develop unique methods for dealing with their sensitivity including the avoidance of cold drinks, some foods, and the use of certain teeth or areas of the mouth. Treatment options for hypersensitivity abound, including both over-the-counter options as well as prescription or professionally-applied products.
Before any treatment is rendered, clinicians must understand the condition’s etiology in each individual patient. Determining why certain patients present with dentinal hypersensitivity while others do not, even though their dentitions may be similar, is often difficult. Some patients with gingival recession and/or exposed dentin complain of hypersensitivity, while others remain symptom free.
BACKGROUND
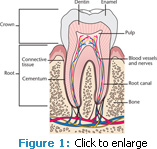
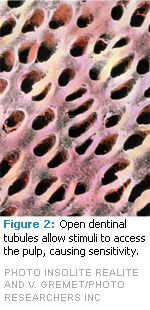
To fully understand the etiology of dentinal hypersensitivity, the anatomy of a tooth must be understood (Figure 1). The root surface is covered by a thin layer of cementum. When gingival recession occurs, the thin layer of cementum is uncovered and can easily be removed—exposing the underlying dentin. In comparison to enamel, dentin contains collagen, is more organic and soluble, and has tubules (Figure 2). The tubules function as a conduit and can allow a stimulus access to the pulp.2 This pathway may be patent and communicate directly with the pulp or it may be completely or partially occluded.2 Factors responsible for tubule occlusion can include the presence of “debris, intratubular inclusions, and/or response dentin.”2 When the pathway to the pulp is unobstructed, dentinal hypersensitivity can occur, which is described as sharp, brief pain that emanates from the exposed dentin in response to thermal, evaporative, tactile, osmotic, or chemical stimuli.3 Dentinal hypersensitivity is different than pain caused by other clinical causations, such as a cracked tooth, fractured restoration, dental caries, or microleakage. Studies have shown that any tooth may present with dentinal hypersensitivity, but it most frequently occurs in canines and premolars and often involves the facial surface.2
In 1900, Gysi first proposed that the pain from dentinal hypersensitivity was caused by fluid in the pulp. In 1963, Brännström described fluid movement into and out of a patent tubule as a source of pain.4 In addition, Brännström found that various pain-producing stimuli, such as air, sugar, or absorbent paper, caused the tubule contents to move outward.5 Sensitive teeth have more patent tubules that are significantly wider than nonsensitive teeth.6 But the fact that a dentinal tubule is open on the surface does not necessarily mean the tooth will be sensitive, as the tubule may be occluded deeper along its pathway to the pulp.2 Also, patient responses to sensitivity are quite subjective, and can vary depending on the source and type of stimulation.7
ROLE OF EROSION
The etiology of dentinal hypersensitivity appears to be multi-factorial. Patients with sensitivity frequently have exceptionally clean teeth and excellent oral hygiene practices, which suggests that dentin hypersensitivity is not related to plaque accumulation.8 Exposure to acid may play a significant role in dentinal hypersensitivity. Erosion is the chemical dissolution of the tooth structure in the absence of bacteria plaque.9 The acid source may be intrinsic, such as gastric acid reflux and/or stomach vapors from belching.
Extrinsic acid sources may be found in foods and beverages either naturally or due to additives, eg, tea contains tannic acid, phosphoric acids are present in colas,10 and citric and maleic acids can be found in fruit, fruit juices, and soft drinks. These acids can demineralize the exposed dentin.
A combination of factors may contribute to the erosive process including the exposure of the root surface following scaling and root planing and periodontal surgery and using an improper brushing technique.11 Toothbrushing immediately following an acid attack can result in a dramatic loss of dentin and should be avoided since the tooth at this time is most vulnerable and weakest.12
IMPORTANCE OF SALIVA
The biofilm/plaque complex is the site of bacterial acid production resulting from their metabolism of sugar. This process of acid production causes the pH to drop and results in the demineralization of tooth structure (enamel and dentin) seen in caries. Saliva and its components, such as bicarbonate, salivary proteins and protein complexes, and calcium phosphate have a strong ability to neutralize and buffer acids and regulate the pH drop.13 In addition, saliva can dilute and help remove erosive agents.13
Dentin more easily dissolves when exposed to acid in comparison to enamel. Critical pH is the pH at which this dissolution begins. Dentin has a critical pH of approximately 6.0 to 6.2.14 The resting pH of the oral environment is in a range of approximately 6.5 to 7.0 and can vary depending on multiple factors including the quantity of saliva that is produced, time of day, patient’s age, and medication history.15 Exposed dentin can be in a continual state of flux, alternating between softening/dissolving and remineralizing.14 Saliva has a key role as a mediator in preventing demineralization and even promoting remineralization.
CURRENT TREATMENT MODALITIES
Before initiating any course of treatment, patients should receive instruction on the proper techniques for maintaining good oral hygiene, and a soft toothbrush should be recommended. Dietary analysis and counseling are also important. Patients should receive information about which foods are acidic and why they should avoid brushing immediately following an acid attack.
Patients should be advised to rinse with water and then allow saliva to act as a buffer and help return the pH to normal. Patients diagnosed with gastroeso phageal reflux disease (GERD), should be evaluated for signs of erosion. Patients with symptoms of erosion, but not yet diagnosed should be referred to their physician for a detailed evaluation and diagnosis. Erosion on the occlusal surfaces is frequently associated with GERD and those with broad/extensive erosion on the lingual surfaces should be evaluated for an eating disorder.
The pain of dentinal hypersensitivity can be addressed by either reducing the excitability of the intradental nerve or by blocking and occluding the tubules.2 There are many products available to address the pain of dentinal hypersensitivity either at home or in the dental office. Each product has a unique chemical composition and method by which the tooth is supplied with the necessary building blocks to encourage remineralization.
Oxalate-based dentifrice is a popular home-based option. Toothpastes containing 5% potassium nitrate or potassium citrate have both demonstrated clinical efficacy, though the effects varied.16 As a result of animal studies, it is thought that potassium has an effect on nerve depolarization and, in high concentrations, has the capability of inhibiting the nerve from repolarizing.17 As such, the pulp is unable to send a pain response.17 However, it is still unclear whether this is the actual mechanism of action by which potassium-containing toothpaste reduce sensitivity.18 Potassium containing toothpastes are also available with fluoride. The addition of fluoride has the added benefit of converting hydroxyapatite (Figure 3) into fluorapatite, which has a critical pH of 4.5, and is more resistant to demineralization in the presence of acid.19
The physical blocking of the tubules can include agents that function solely in a mechanical manner and those that block the tubules through remineralization. Fluoride varnish is the most frequently used chairside treatment for sensitivity and it occludes dentin tubules, creating a barrier to painful stimuli. Stannous fluoride and sodium fluoride gels also address the pain of sensitivity by blocking the dentinal tubules. Bonding agents and sealants, applied in the dental office, can mechanically block and occlude the dentinal tubules. Adhesive resins form a hybrid layer, which may seal the dentinal tubules. Dentin bonding agents may remove the smear layer, etch the surface of dentin, and create a hybrid layer, thus occluding the tubules.20,21
Pro-Argin™ Technology is a calcium-based remineralizing product containing an arginine bicarbonate/calcium carbonate compound that is applied chairside for the treatment of dentinal hypersensitivity.22 The highly soluble arginine bicarbonate interacts with the poorly soluble calcium carbonate component to plug the dentinal tubules.23 The arginine component is designed to enhance the adhesion of the calcium plug and reduce its solubility.
Created by the American Dental Association Paffenbarger Research Center in 1991, amorphous calcium phosphate (ACP) releases calcium and phosphate ions in the presence of saliva to encourage remineralization. It occludes the dentinal tubules and provides a reservoir of calcium and phosphate ions in saliva.24 Recaldent™ is another ingredient used in desensitizing products. It includes a casein phospho-peptide (CPP) with ACP. This desensitizing and remineralizing product allows for an increase in the biofilm’s content of calcium and phosphate.25 In this formulation, the CPP is designed to stabilize the ACP prior to reaching the tooth surface.
This increase in calcium and phosphate is thought to help in the remineralization of the root surface.NovaMin® has the chemical name of calcium sodium phosphosilicate and consists of calcium, phosphorus, silica, and sodium. In a moist environment, these ions are released in an extremely high concentration and combine to form a hydroxycarbonate apatite (HCA) layer. In vitro studies have shown that dentin treated with NovaMin® resulted in tubule occlusion, and that the dentin tubules were more acid resistant and demonstrated less enlargement following citric acid exposure.26
A new tri-calcium phosphate (TCP) technology is included in fluoride varnish and prescription dentifrice. Tri-calcium phosphate is manufactured using a special process in which tri-calcium phosphate and organic ingredients are combined to create a bioactive tri-calcium phosphate. These products are able to release active fluoride and calcium that can either remineralize the tooth or desensitize and block the tubules. In vitro studies have shown that toothpastes containing functionalized TCP technology provided complete tubule occlusion.27
Lasers are also used to treat dentinal hypersensitivity but their mechanism of action for this application is not clear. Some studies show that lasers can occlude the dentinal tubules, while others found they block the movement of fluid in the tubules.28,29 More research is needed to support this usage.
CONCLUSION
Before undergoing any course of treatment, it is very important to engage patients in a detailed discussion about their sensitive teeth and to perform a thorough clinical examination and diagnosis. Although, dentinal hypersensitivity can have multiple overlapping etiologies, each patient should be assessed on an individual basis and a personalized treatment plan developed. Development of dentinal hypersensitivity is closely tied to both dentinal erosion and abrasion, but the maintenance of the exposed tubules appears to be determined heavily by saliva. The protective and remineralizing nature of the dental biofilm/pellicle appears to function similarly in both dentin hypersensitivity and caries. Several products on the market have been proven to be helpful. Minimally invasive choices should always be attempted first before consideration is given toward operative dentistry.
REFERENCES
- Chabanski MB, Gillam DG, Bulman JS, Newman HN. Clinical evaluation of cervical dentine sensitivity in a population of patients referred to a specialist periodontology department: a pilot study. J Oral Rehabil. 1997;24:666–672.
- Orchardson R, Gangarosa LP, Holland GR, et al. Consensus report, dentine hypersensitivity into the 21st century. Archs Oral Biol. 1994;39:635–715.
- Holland GR, Narhi MN, Addy M, Gangarosa L, Orchardson R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J Clin Periodontol. 1997;24:808–813.
- Brännström M. A hydrodynamic mechanism in the transmission of pain-produced stimuli through the dentine. In: Anderson DJ, ed. Sensory Mechanisms in Dentine. Oxford: Pergamon Press; 1963:73–79.
- Brännström M, Linden LA, Astrom A. The hydrodynamics of the dental tubule and of pulp fluid. A discussion of its significance in relation to dentinal sensitivity. Caries Res. 1967;1:310–317.
- Absi EG, Addy M, Adams D. Dentine hypersensitivity. A study of the patency of dentinal tubules in sensitive and nonsensitive cervical dentine. J Clin Periodontol. 1987;14:280–284.
- Kleinberg I, Kaufman HW, Wolff MS. Measurement of tooth hypersensitivity and oral factors involved in its development. Arch Oral Biol. 1994;39:63S–71S.
- Addy M, Mostafa P, Newcombe RG. Dentine hypersensitivity: the distribution of recession sensitivity and plaque. J Dent. 1987;15:242–248.
- Hannig M, Hess NJ, Hoth-Hannig W, De Vrese M. Influence of salivary pellicle formation time on enamel demineralization—an in situ pilot study. Clin Oral Investig. 2003;7:158–161.
- West NX, Hughes JA, Addy M. The effect of pH on the erosion of dentine and enamel by dietary acids in vitro. J Oral Rehabil. 2001;28:860–864.
- Volpe AR, Monney R, Zumbrunnen C, Stahl D, Goldman HM. A long term clinical study evaluating the effect of two dentifrices on oral tissues. J Periodontal. 1975;46:113–118.
- Absi EG, Addy M, Adams D. Dentine hypersensitivity—the effect of toothbrushing and dietary compounds on dentine in vitro: an SEM study. J Oral Rehabil. 1992;19:101–110.
- Kleinberg I. Formation and accumulation of acid on the tooth surface. J Dent Res. 1970;49:1300–1317.
- Vanuspong W, Eisenburger M, Addy M. Cervical tooth wear and sensitivity: erosion, softening and rehardening of dentine; effects of pH, time and ultrasonication. J Clin Peridontol. 2002;29:351–357.
- Palmer, CA. Diet and Nutrition in Oral Health. 2nd ed. Upper Saddle River, NJ: Pearson/Prentice Hall; 2007:274.
- Chesters R, Kaufman HW, Wolff MS, Huntington E, Kleinberg I. Use of multiple sensitivity measurements and logit statistical analysis to assess the effectiveness of a potassium citrate-containing dentifrice in reducing dentinal hypersensitivity. J Clin Periodontol. 1992;19:256–261.
- Kim S. Hyperensitive teeth: desensitization of pulpal sensory nerves. J Endod. 1986;12:482–485.
- Poulsen S, Errboe M, Lescay MY, Glenny AM. Potassium containing toothpastes for dentine hypersensitivity. Cochrane Database Syst Rev. 2006;3:CD001476.
- Summitt, JB. Fundamentals of Operative Dentistry: A Contemporary Approach. 3rd ed. Chicago: Quintessence Publishing; 2006:408.
- Baysan A, Lynch E. Treatment of cervical sensitivity with a root sealant. Am J Dent. 2003;16:135–138.
- Dondi dall’Orologio G, Lorenzi R, Anselmi M, Opisso V. Dentin desensitizing effects of Gluma Alternate, Health-Dent Desensitizer and Scotchbond Multi-Purpose. Am J Dent. 1999;12:103–106.
- Panagakos F, Schiff T, Guignon A. Dentin hypersensitivity: Effective treatment with an inoffice desensitizing paste containing 8% arginine and calcium carbonate. Am J Dent. 2009;22:3A–7A.
- Kleinberg I. SensiStat. A new saliva-based composition for simple and effective treatment of dentinal sensitivity pain. Dent Today. 2002;21:42–47.
- Geiger S, Matalon S, Blasbalg J, Tung M, Eichmiller FC. The clinical effect of amorphous calcium phosphate (ACP) on root surface hypersensitivity. Oper Dent. 2003;28:496–500.
- Shen P, Cai F, Nowicki A, Vincent J, Reynolds EC. Remineralization of enamel subsurface lesions by sugar-free chewing gum containing casein phosphopeptide-amorphous calcium phosphate. J Dent Res. 2001;80:2066–2070.
- Wang Z, Jiang T, Sauro S, et al. The dentine remineralization activity of a desensitizing bioactive glass-containing toothpaste: an in vitro study. Aust Dent J. 2011;56:372–381.
- Mackey, AC, Karlinsey, RL, Gidley, J, Stookey, G, Pfarrer, A. In vitro assessment of dentin tubule occlusion by hypersensitivity dentifrices. J Dent Res. 2009;88(Spec Iss A):Abstract 1935.
- Corona SA, Nascimento TN, Catirse AB, Lizarelli RF, Dinelli W, Palma-Dibb RG. Clinical evaluation of low-level laser therapy and fluoride varnish for treating cervical dentinal hypersensitivity. J Oral Rehabil. 2003;30:1183–1189.
- Clayton DR, McCarthy D, Gillam DG. A study of the prevalence and distribution of dentine sensitivity in a population of 17–58-year-old serving personnel on an RAF base in the Midlands. J Oral Rehabil. 2002;29:14–23.
From Dimensions of Dental Hygiene. April 2012; 10(4): 20, 22, 24, 26.