 NUTTHASETH VANCHAICHANA / ISTOCK / GETTY IMAGES PLUS
NUTTHASETH VANCHAICHANA / ISTOCK / GETTY IMAGES PLUS
Quenching Thirst or Demineralizing Tooth Enamel?
The pH of beverages significantly impacts the health of enamel.
Dental enamel is the hardest and most highly mineralized substance in the human body. A crystalline latticework composed of a variety of minerals—predominantly hydroxyapatite—the enamel structure is a product of the demineralization and remineralization process. Dental biofilm adjusts the tooth surface pH, which affects the demineralization/remineralization process. Consumption of fermentable carbohydrates, especially sucrose, provides the substrate for the cariogenic microorganisms in the biofilm to form organic acids.1 When acids lower the pH of the dental biofilm, the demineralization process begins to break down tooth enamel. The loss of calcium and phosphates from the surface and subsurface enamel into the pellicle and biofilm result in white lesions in the enamel.1 Consequently, if dental biofilm is not routinely and effectively removed from tooth surfaces, the demineralized white lesions continue to develop into caries lesions.
Demineralization is the process of removing mineral ions from the hydroxyapatite crystals of tooth tissue—namely, enamel, dentin, and cementum. It is one of the leading causes of dental erosion and caries lesions. The restoration of these mineral ions to the hydroxyapatite crystals is called remineralization. Both processes occur on the tooth surface.2 Demineralization may be caused by a variety of intrinsic and extrinsic factors. Intrinsic factors are commonly due to diseases while extrinsic factors include diet and medication usage.2 Beverage consumption is an extrinsic factor of particular interest.
Variety of Drink Options
Carbonated soft drinks are second in popularity only to bottled water in the United States.3 Soft drinks are generally sweet, nonalcoholic effervescent beverages that encompass a wide range of beverages including soda, fruit juices, sports drinks, and energy drinks. While 22% of Americans believe that carbonated soft drinks are enjoyable and refreshing beverages,3 their consumption causes many deleterious effects on overall and oral health, specifically by demineralizing tooth enamel.
Many soft drinks contain carbon dioxide, which produces carbonation. Other ingredients include water, sugar (sucrose, glucose, and fructose), artificial sweeteners, acid (citric acid, malic acid, and phosphoric acid), fruit juice, preservatives, flavorings, and colors.4 Many are high in sugar and acid content. Their pH scale ranges from 0 to 14 (7 is neutral). A liquid’s pH level is the measurement of its free-flowing electrically charged particles, indicating acidity (below 7) or alkalinity (above 7).5 Table 1 lists the pH levels of many popular drinks.6 Subsequently, the impact of a beverage’s pH on demineralization is an important factor to consider during each patient’s assessment.
In addition to carbonated beverages, the regular consumption of acidic beverages is an emerging problem for children, teenagers, and adults. The dramatic increase in consumption of acidic soft drinks, fruit juices, fruit drinks, sports drinks, and carbonated beverages is now thought to be the leading cause of dental erosion observed among children and adolescents.7 A literature review of dental erosion in children indicates its prevalence may range from 10% to 80%.8 Primary teeth with their thinner enamel layer are more susceptible to rapid demineralization into dentin, leading to exposure of the dental pulp. Demineralization of tooth enamel necessitates restorative treatment to replace lost tooth structure, eliminate dental pain, and restore function and esthetics.8
Spiked and hard seltzers have become popular among adults. These alcoholic drinks are typically created from malt liquor or fermented cane sugar with added artificial fruit flavors. Malt liquor is a strong lager or ale in which sugar, corn, or other additives are combined with the malted barley to increase the total amount of fermentable sugars. Additionally, regular consumption of acidic drinks, including wine, increases the acidity of the oral cavity. As a result, tooth enamel becomes more susceptible to demineralization. 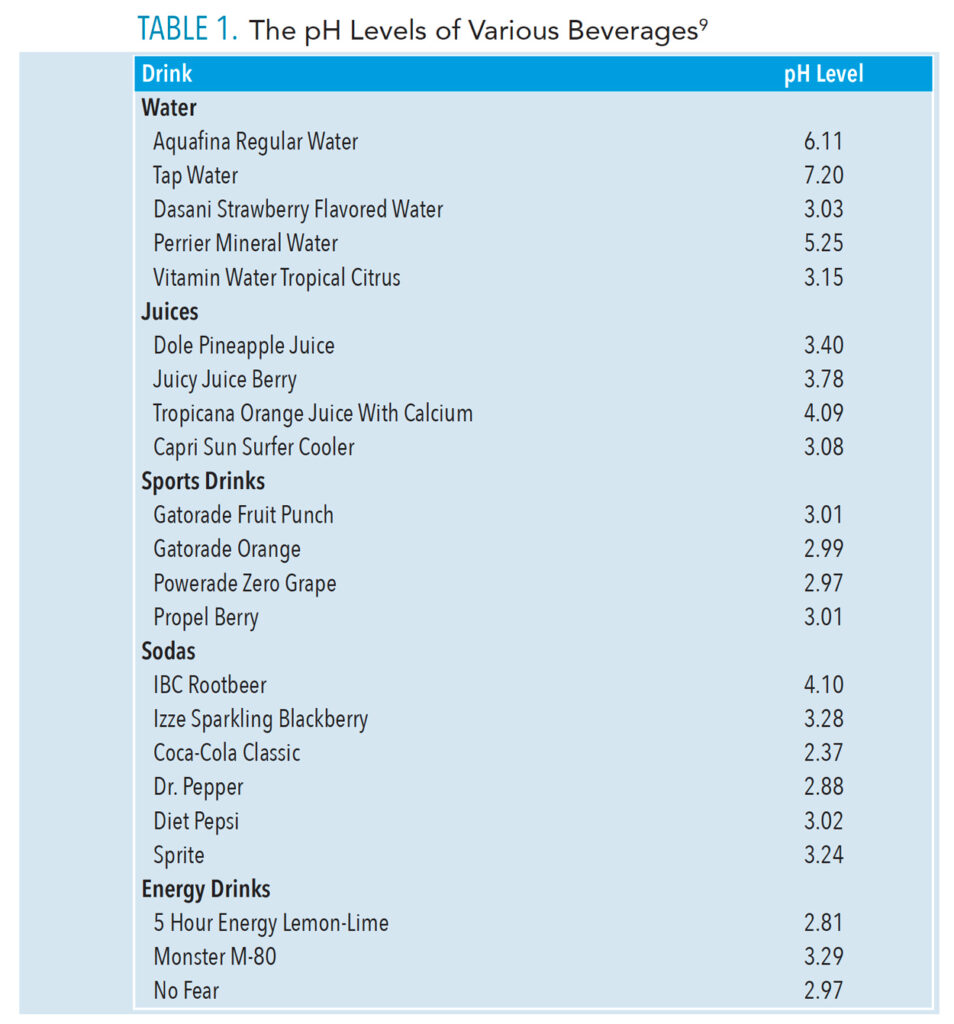
Risk for Xerostomia
Tooth enamel demineralization is further assisted by a decrease in salivary secretion, leading to a diminishment of buffering capacity.8 Saliva is essential for the dilution and buffering of hydrogen ions in the oral cavity.6 While key to prohibiting demineralization by diluting and buffering extrinsic acids, saliva also provides glycoproteins that coat the tooth surface as the protective acquired pellicle.6 However, when acidic beverage consumption is excessive, saliva offers limited protection from erosion.
Importance of pH
When the pH of the oral cavity reaches 5.5, the hydroxyapatite in dental enamel starts to dissolve.4 As the pH in the oral cavity drops below 4.0, the tooth surface erodes. With each unit of decrease in pH, a 10-fold increase occurs in enamel solubility. As the pH approaches 2.0, there is a 100-fold increase in enamel demineralization. The consumption of beverages with higher concentrations of available hydrogen ions (pH < 4.0) results in the immediate softening of the tooth surface, causing the tooth to become susceptible to abrasion and attrition.6 The pH of most soft drinks ranges from 2.5 to 3.5, with an average of 3.44 putting them well below the critical pH.4 As tooth structures begin to demineralize in the presence of low pH, bacteria easily colonize. This phenomenon—in addition to acid formation—enables pathogens to penetrate into the dentin, ultimately, causing erosion or decay. Furthermore, the risk for caries is greater when highly acidic drinks are consumed.2 Thus, educating patients on the pH of different beverages and how their consumption contributes to tooth decay is important.
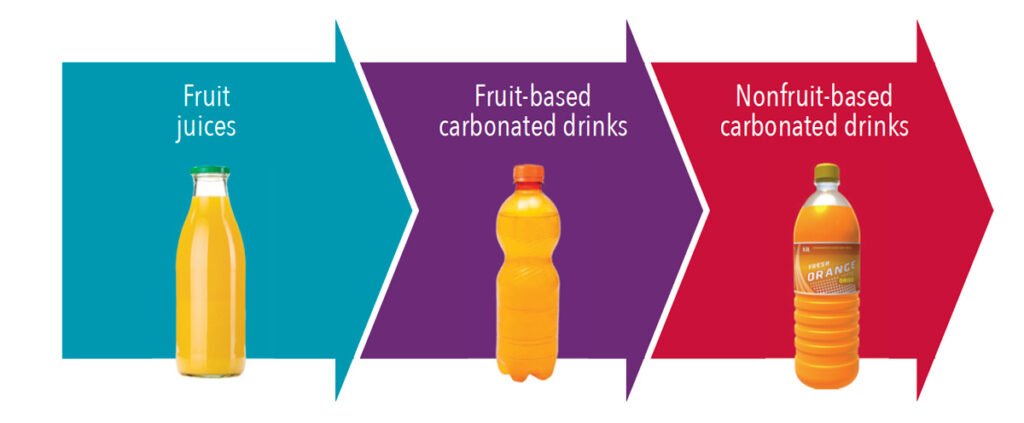
The elimination of extremely erosive drinks (pH < 3.0), minimizing erosive drinks (pH 3.0 – 3.99), and substituting drinks with a pH ≥ 4.0 is helpful in the prevention of erosion.8 Moreover, carbonated drinks have a lower pH than fruit juices (Figure 1).9
A knowledge of the pH of beverages should be part of preventive and remineralization treatment planning for patients with signs of clinical demineralization. Oral health professionals need to be able to provide dietary suggestions when counseling patients on the damaging effects of acidic beverages.
Remineralization Process
Remineralization is a natural repair mechanism used to restore minerals in ionic forms to the hydroxyapatite crystal lattice of the tooth. It occurs under near neutral physiological pH conditions whereby calcium and phosphate mineral ions are redeposited within the caries lesion from saliva and plaque fluid. This results in the formation of newer hydroxyapatite crystals that are larger and more resistant to acid dissolution.
Fluoride is considered the standard therapy for tooth remineralization. It functions by forming a fluorohydroxyapatite complex, making the tooth surface stronger and more resistant to decay than hydroxyapatite alone. Fluoride also acts as a catalyst in the remineralization of enamel with phosphate ions present in the saliva.2 Widely used in over-the-counter oral health products, fluoride also has in-office professional uses. For example, the application of fluoride varnish enhances tooth remineralization in children. Prescription fluoride toothpaste is effective among patients with many incipient caries lesions.
Other remineralizing agents may also achieve positive results. A recent study found that immersion in beverages modified with casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) paste remineralized enamel.10 The CPP was able to bind to the apatite crystal faces in the surface of enamel lesions, keeping the diffusion pathways open to allow ions to penetrate more deeply. This resulted in remineralization throughout the body of the lesion, rather than just the surface layer. The application of CPP-ACP paste may enhance remineralization after an erosive challenge, thus, offering some protection for patients at risk for erosion. CPP-ACP paste may even be added to certain beverages to further prevent tooth erosion.10
Another study found that grape seed extracts rich in proanthocyanidins inhibit the demineralization of caries lesions in both the enamel and dentin by reducing collagen degradation and possibly inhibiting the acid production by Streptococcus mutans.11 Beverage manufacturers may want to consider adding grape seed extracts to their drinks to help reduce tooth demineralization. Increasing the concentration of calcium and/or phosphate in beverages may help teeth withstand lower pH values before demineralizing.2
The remineralization process restores minerals in ionic forms to the hydroxyapatite crystal lattice of the tooth.
Putting Knowledge Into Practice
Oral health professionals should discuss the consumption of acidic beverages during the patient’s comprehensive evaluation. This is especially true for children and adolescents who have a vulnerable mixed dentition and may be unaware of the demineralization properties of popular drinks.12 The addition of fluoride mouthrinses into oral hygiene regimens will help to remineralize the susceptible areas in dental enamel. Using products containing calcium phosphate technologies may also support remineralization. Rinsing with water after consuming demineralizing drinks in order to neutralize the acidogenic properties of the beverage may also help prevent demineralization.12 Ensuring patients have a broad understanding of how best to protect their dentition is integral to maintaining a lifetime of oral health.
References
- Roberts M, Wright T. The dynamic process of demineralization and remineralization. Dimensions of Dental Hygiene. 2009;7(7):16–21.
- Abou Neel EA, Aljabo A, Strange A, et al. Demineralization-remineralization dynamics in teeth and bone. InJ J Nanomedicine. 2016; 19:4743–4763.
- Tarantino O. 12 Most popular drinks in America—ranked. Eat This, Not That! Available at: eatthis.c/m/most-popular-drinks-america. Accessed March 16, 2021.
- Tahmassebi JF, BaniHani A. Impact of soft drinks to health and economy: a critical review. Eur Arch Paediatr Dent. 2020;21:109–117.
- Cirino E. What pH should my drinking water be? Healthline. Available at: healthline.com/health/ph-of-drinking-water. Accessed March 16, 2021.
- Reddy A, Norris DF, Momeni SS, Waldo B, Ruby JD. The pH of beverages in the United States. J Am Dent Assoc. 2016;147:255–263.
- Lussi A, Jaeggi T. Dental erosion in children. Monogr Oral Sci. 2006;20:140–151.
- Taji S, Seow WK. A literature review of dental erosion in children. Aust Dent J. 2010;55:358–367.
- Cheng R, Yang H, Shao MY, Hu T, Zhou XD. Dental erosion and severe tooth decay related to soft drinks: a case report and literature review. J Zhejiang Univ Sci B. 2009;10:395–399.
- Rai N, Sandhu M, Sachdev V, Sharma R. Evaluation of remineralization potential of beverages modified with casein phosphopeptide-amorphous calcium phosphate on primary and permanent enamel: a laser profiler study. Int J Clin Pediatr Dent. 2018;11:7–12.
- Silva AP, Gonçalves RS, Borges AF, Bedran-Russo AK, Shinohara MS. Effectiveness of plant-derived proanthocyanidins on demineralization on enamel and dentin under artificial cariogenic challenge. J Appl Oral Sci. 2015;23:302–309.
- Marsh L. Reducing demineralization risk. Dimensions of Dental Hygiene. 2017;15(5):18–20.
From Dimensions of Dental Hygiene. April 2022;20(4):16,18,21.

