Promote Periodontal Health
The use of nonsurgical periodontal therapy to treat chronic periodontitis is standard in oral health care, and new developments may improve its efficacy in the future.
This course was published in the February 2013 issue and expires February 2016. The author has no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the methods of nonsurgical therapy, as well as their potential and limitations in achieving oral health.
- Identify other factors that affect the preservation of the dentition.
- List current and future trends in periodontal therapy.
INTRODUCTION

As dental professionals, we strive to achieve optimal outcomes for all of our patients. Despite our very best efforts, however, there will be some patients who do not respond as we expected, and their periodontal health will continue to decline. There are many factors that may be responsible for this poor response, including systemic, local, environmental, and lifestyle factors. In 2012, data on the prevalence of periodontitis in the United States population was published in the Journal of Dental Research. This publication confirmed what many dental professionals had already observed: almost half of American adults have mild, moderate, or severe periodontitis. In this article, “Promote Periodontal Health,” Kristi M. Soileau, DDS, MEd, provides a timely review of the benefits and limitations of nonsurgical periodontal therapy and the rationale for therapy choices.
The Colgate-Palmolive Company is delighted to have provided an unrestricted educational grant to support the first article of this educational series on implementing a team approach to improve periodontal outcomes in collaboration with the American Academy of Periodontology.
— Barbara Shearer, BDS, MDS, PhD
Director of Scientific Affairs
Colgate Oral Pharmaceuticals
While the inflammatory components of plaque Induced-gingivitis can be managed relatively Efficiently with appropriate self-care and; regular maintenance visits, the treatment of chronic periodontitis is more challenging. The 2009-2010 National Heath and Examination Survey reports that 47.2% of adults have periodontitis. 1 A majority of patients with periodontitis may be managed by a plaque control and maintenance program, however, more aggressive treatment modalities—including both nonsurgical and surgical procedures—are more likely indicated.2
THE CRUX OF THE CHALLENGE
More than one type of bacteria may be responsible for the clinical signs and symptoms of periodontitis. Therefore, successful treatment depends on an accurate diagnosis, which is based on comprehensive data collection of clinical and, when necessary, microbial findings.3 For complete debridement of deep pockets, furcations, or areas with root surface irregularities, surgical access may be necessary. For areas that are accessible via nonsurgical means, dental professionals must be able to support the treatment modality selected and then use follow-up monitoring to ascertain whether the treatment is successful or if the original treatment plan needs modification. Nonsurgical strategies include supragingival and subgingival irrigation, scaling and root planing, and adjunctive antibiotic therapy.
SUPRAGINGIVAL AND SUBGINGIVAL IRRIGATION
The primary theory behind supragingival irrigation is to flush away bacteria coronal to the gingival margin, diminishing the potential for gingivitis or decreasing existing gingival inflammation. In contrast, subgingival irrigation attempts to directly reduce the pocket microflora to prevent initiation of periodontal diseases or to facilitate their reduction. Research supports the use of supragingival (sulcular) irrigation, with water or medicaments, by patients as part of their oral hygiene routines.
However, little data exist to support the theory that a single episode of in-office subgingival irrigation increases the immediate impact or duration of root planing efficacy. Likewise, there is limited information to suggest that multiple in-office irrigation appointments provide a substantial benefit beyond root planing, unless the root planing was poorly done.4 Also unclear is at what frequency and duration irrigation should be employed.3
SCALING AND ROOT PLANING
As with all other modalities, scaling and root planing must be accompanied by oral hygiene instruction, as well as monitoring the patient’s hygiene performance from visit to visit. The goal of scaling and root planing is the removal of soft and hard debris from both the coronal as well as the radicular surfaces of the tooth as thoroughly and meticulously as possible within the physical and anatomical limitations of each individual patient. The removal of plaque and calculus is more difficult in deeper pockets and areas that are more challenging to reach and diagnose, such as furcations.
In a recent study assessing the effectiveness of scaling and root planing in the reduction of preterm birth and low-birthweight risks, scaling and root planing reduced the risk of preterm birth in pregnant women with periodontitis among those who were already predisposed to preterm birth.5 Study results are mixed regarding the effect scaling and root planing may have on adverse pregnancy outcomes. Therefore, further research is needed to define those groups in which risk reduction might be most effective.
Thorough scaling and root planing is highly effective in the treatment of chronic periodontitis and is the standard approach to nonsurgical therapy.6 It is often necessary, however, to continue therapy with a surgical approach when root planing does not achieve the periodontal stability desired.
ADJUNCTIVE ANTIBIOTIC THERAPY
Oral bacterial flora are relatively complex, and vary significantly from one patient to another. Radiographs and periodontal probing can detect past destruction, but not necessarily when it occurred. Therefore, it is difficult to consistently recognize patients presenting with periodontal diseases who may require or benefit from the adjunctive use of an antibiotic.2
There are conflicting data on the ability of local drug delivery to enhance results of scaling and root planing at deep probing sites, and there is limited information regarding its capability to inhibit disease progression or enhance osseous repair in infrabony defects.7 Patients who have not responded favorably to previous therapy may benefit from antibiotic therapy, although it is not a substitute for thorough instrumentation of a problematic site.
In a clinical trial of patients with type 2 diabetes, results showed that the local delivery of 0.5% azithromycin enhanced the clinical outcome of scaling and root planing.8 In smokers, subgingivally-placed minocycline in conjunction with scaling and root planing reduced bacterial levels greater than with scaling and root planing alone.9
The concept that a locally delivered antibiotic (LDA) into the periodontal pocket achieves a greater, more potent concentration of drug than is available with systemic delivery is very attractive. The amount of drug delivered often exceeds the equivalent of 1 mg/ml (1000 ?g/ml), which is considered bactericidal for most bacteria that exhibit resistance to systemically-delivered concentrations. Equally important, LDAs have a negligible impact on the microflora residing in other regions of the body.2
A statement from the American Academy of Periodontology (AAP) in 2006 asserted that clinicians may consider the use of LDAs in patients with chronic periodontitis as an adjunct to scaling and root planing when localized recurrent and/or residual pocket depth is greater or equal to 5 mm with inflammation remaining after conventional therapies have been tried. Therapies other than LDAs should be considered when multiple sites with pocket depths greater or equal to 5 mm exist in the same quadrant, when the use of LDAs failed to control periodontitis (eg, reduction of pocket depth), or when anatomical defects are present (eg, intrabony defects).6

RE-EVALUATION, MAINTENANCE, AND ORAL HYGIENE
The backbone of periodontal treatment consists of mechanical removal of bacterial deposits and calculus from the subgingival environment along with a strict regimen of plaque control. The main clinical parameter to indicate successful treatment should be tooth loss/ maintenance over the years, but in the short term, a 1-month post-root planing evaluation of pocket reduction, attachment gain, and bleeding on probing is essential in assessing a patient’s status.10
The key to success of any nonsurgical or surgical therapeutic program is a continued monitoring of patient status and self-care compliance.11 This is commonly accomplished by determining a recare frequency that best suits the patient and performing not only the necessary dental cleaning but also examination of clinical parameters such as probing depths, bleeding and exudate status, and oral hygiene score. This should also include a soft tissue exam, dental exam, and radiographic review. Therefore, periodontal maintenance is not synonymous with prophylaxis. Table 1 lists the goals of periodontal maintenance.
Studies show that patients who maintain regular maintenance intervals experience less attachment loss than those who receive less maintenance12,13 or none at all.14 In office periodontal maintenance at 1-month to 4-month intervals can be effective in maintaining most patients.15,16 Although an interval of 3 months between appointments for patients with a history of periodontitis appears to be effective, this can vary, depending on patient compliance, as well as the clinical judgment of the dental professional. When new or recurring periodontal diseases appear, additional diagnostic and treatment procedures must be considered. Long-term control of disease depends on active periodontal maintenance care and appropriate additional therapy, if indicated.17
OTHER CONSIDERATIONS IN PRESERVING DENTITION
Besides evaluating intraoral conditions, consideration must be given to other factors, such as systemic factors, genetic predisposition, pregnancy, teeth anatomy, occlusion, and smoking, in assisting the patient to achieve periodontal health. Due to the ulceration of the gingival sulcus, chronic periodontitis can contribute not only to systemic bacterial dissemination, but also to widespread inflammation throughout the body. Chronic periodontitis can act as a metabolic stresser for diabetes control.10 Conversely, patients with poorly controlled diabetes exhibit more periodontal disease.18,19 High levels of C-reactive protein are associated with both diabetes and cardiovascular diseases. Poor oral health is also associated with coronary artery disease.20 Although there is controversy regarding the direct impact of periodontal therapy on systemic inflammation, there is consensus regarding the clinical and microbiologic benefits of periodontal therapy.21
The number and type of bacteria required to exceed an individual’s disease threshold defines host susceptibility, which may be influenced by a number of genetic and nongenetic determinants. Although results from family studies suggest that environmental factors appear to be the major determinants of variance in adult periodontitis, data from a Minnesota Twin Periodontal Study in 1994 indicated that both genetic and environmental factors influence disease. Furthermore, comparisons between reared-together and reared-apart adult monozygous twins indicated that early family environment had no appreciable influence on probing depth and attachment loss measured in adults.2 More recently, research further confirms that specific bacteria are essential for disease initiation, but genetic and environmental host modifiers appear to influence disease severity. For example, analysis of disease expression in twins suggests that approximately 50% of variance in clinical phenotype is explained by genetics.22
Nearly 13% of all births in the United States occur before 37 weeks of gestation, and preterm birth is the leading cause of perinatal morbidity and mortality.23 For more than 50 years, maternal infection and inflammation have been thought to contribute to poor pregnancy outcomes. While the associations between preterm birth and infections such as chorioamnionitis, bacterial vaginosis, and urinary tract infections, are well-established, there also is evidence that “distant” infections, including periodontal diseases may also increase a woman’s risk for preterm birth.24
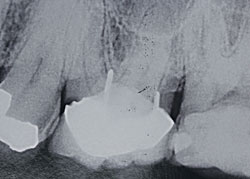
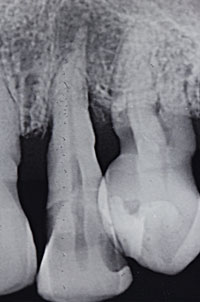
Plaque control can be more difficult when considering factors such as defective restorations (eg, around overhangings and open margins), carious areas, furcation involvement, and deeper probing depth, as all can cause accessibility problems. These issues should be evaluated and addressed at the maintenance visit. Another localized consideration is trauma from occlusion. Occlusal trauma can occur in conjunction with or independent of inflammatory diseases. The ability of the attachment apparatus to withstand a less than ideal occlusion may be compromised by periodontal inflammation. Thus, trauma from occlusion may accelerate attachment loss in a patient with active chronic periodontitis (Figure 1 and Figure 2).25 However, removal of occlusal interferences and the subsequent reduction of tooth mobility will not typically improve periodontal conditions already present.26
A little more than 20% of the United States population smokes, according to the federal Centers for Disease Control and Prevention. Periodontal treatment in smokers is challenging. Smokers will respond less favorably to both nonsurgical and surgical periodontal treatment than nonsmokers. Both heavy and light smokers have less pocket depth reduction and less attachment gain following active treatment throughout a supportive periodontal phase.10,27,28 Additionally, research shows that quitting smoking reduces probing depths at 12 months following cessation.29 Practitioners need to offer proper counseling on the relationship between smoking and periodontal diseases.
THE CASE FOR NONSURGICAL VS SURGICAL TREATMENT
There are many instances in which a patient may require surgery, yet is unable to undergo the necessary therapy due to psychological, physiological, or financial reasons. In these cases, great care must be given to maintain the patient’s status without further deterioration where possible. Understanding the limitations of nonsurgical therapy and referral to a periodontist are the best treatments for more advanced cases.
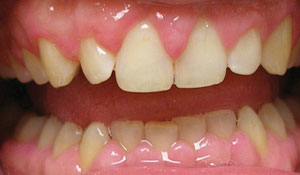
In patients with fibrous gingival overgrowth, whether from pregnancy,30 idiopathic or medication-induced12 causation, modification of tissue topography, will require surgical intervention to create a maintainable oral environment (Figure 3). In patients for whom medication has created this issue, a consultation with the treating physician to investigate the possibility of alternative drug therapy is advised.
In general, surgical therapy should be considered for patients who require better access for removal of etiologic factors, reduction of deep probing depths, or regeneration or reconstruction of lost periodontal tissues.31–33
IS DENTAL LASER THERAPY NONSURGICAL TREATMENT?
Although some see the laser as an alternative to traditional surgical therapy since it utilizes a wavelength of light to target diseased tissue rather than a conventional scalpel blade, it is nonetheless a form of surgery34–39 and should not be categorized as nonsurgical therapy. Additionally, despite the large number of published studies, the usefulness of lasers for chronic periodontitis treatment is controversial. Studies show that lasers, such as Nd:YAG and Er:YAG, achieve a reduction in the number of periodontal pathogens and pocket depth. However, the evidence is currently insufficient to support greater benefits of lasers over traditional scaling and root planing or additional benefits when used in conjunction with scaling and root planing in the treatment of chronic periodontitis.40
TRENDS IN NEW PERIODONTAL THERAPIES
Breakthroughs in understanding the mechanisms that mediate and resolve tissue destruction in periodontal diseases are on the horizon, and these discoveries may provide new tools for regeneration of periodontal structures as well as to explain associations between periodontitis and other chronic inflammatory diseases. The results of fundamental microbiological research will lead to innovative methods of identifying and altering pathogenic biofilms.41
The recently discovered cytokine, interleukin-29 (IL-29), has antiviral properties, and its production is induced by herpes viruses. In a study that compared individuals with healthy periodontium to patients with chronic periodontitis and those with aggressive periodontitis, results showed that the antiviral IL-29 levels were highest among the gingival crevicular fluid of patients with aggressive periodontitis and lowest in the healthy subjects, after scaling and root planing was completed. As such, IL-29 deserves further investigation as a potential therapeutic agent in treating periodontitis nonsurgically.42
Certain molecules can serve as potential biomarkers that may aid in understanding cellular biological principles, such as cell-matrix dynamics and homeostasis of periodontal breakdown. The endpoint goal is to determine the patient’s susceptibility to regression due to periodontal pathogenic exposure, and this may assist clinicians in identifying contributing factors that could then be targeted in an effort to control the inflammatory process.43

Photodisinfection is the targeting and elimination of the bacteria most responsible for the progression of periodontal diseases with the use of a methylene blue dye that is injected into the periodontal pocket. The dye binds to the lipopolysaccharides and lipids found on the cell walls of both Gram-negative and Grampositive bacteria. Gram-negative bacteria take up the methylene blue stain faster, due to their thicker cell walls. The use of a nonthermal diode laser produces photons that hit the dye molecules of the cell walls. The oxygen molecules surrounding the dye then lose an electron and thus, become free radicals, which are toxic to the bacterial cell wall.44
Vaccination is a process that induces specific immune resistance to a bacterial or viral infectious disease. In considering a vaccine against periodontal diseases, the complexity of the periodontopathic bacteria might be a problem in determination of specific antigens. Among some 300 species of bacteria involved in subgingival plaque, five to seven species have been implicated in the possible etiology of periodontitis, but two species, Porphyromonas gingivalis and Tannerella forsythia, might play more important roles as primary pathogens.45 Table 2 lists the three ways that vaccination can be accomplished. Although a periodontal vaccine could have far reaching health benefits, 45 an insufficient quantity and quality of evidence hinders this development.46
Attempts to identify markers of future disease go back several years. For example, the presence of visible plaque and calculus, as one example of a hypothesized marker, was long assumed to predict future clinical attachment loss or bone loss, but studies have shown that plaque and calculus by themselves do not predict future disease to any useful extent. Rather, the subgingival presence of specific pathogens have been shown to offer a moderate degree of predictability.47,48 Additionally, risk factors for attachment loss were found to be increased by age, smoking, diabetes, and the presence of subgingival P. gingivalis and T. forsythia, after controlling for gender, socioeconomic status, education, and oral hygiene status.49
Another study by the same author related increased age, smoking, race, and presence of P. gingivalis and T. forsythia as risk indicators for alveolar bone loss.50 While risk prediction is still not a precise science in periodontology, enough advances have been made to permit development of a calculator to help assess a patient’s risk of disease. Refinement of risk prediction models in the future will give practitioners an ever-improving evidence base upon which to select treatment.10,47
SUMMARY
The AAP states in its treatment guidelines that periodontal health should be achieved in the least invasive and most cost-effective manner possible for each patient.51 When nonsurgical therapy does not achieve periodontal health and stability, surgery may be indicated to restore and regenerate damaged periodontal tissues. Regardless of the treatment method necessary, frequent re-evaluation and careful monitoring allow for early intervention in the disease state, in order to reverse or arrest the progression of periodontal disease as efficaciously as possible.1
ACKNOWLEDGEMENTS
CREDIT: A. DOWSETT, HEALTH PROTECTION AGENCY/SCIENCE PHOTO LIBRARY
REFERENCES
-
- Drisko CH. Nonsurgical periodontal therapy. Periodontol 2000.2001;25:77–88.
- Walker C, Karpinia K. Rationale for use of antibiotics in periodontics.J Periodontol. 2002;73:1188–1196.
- Stein M. A literature review: oral irrigation therapy. The adjunctive roles for home and professional use. Probe.1993;27:18–25.
- Greenstein G; Research, Science and Therapy Committee ofthe American Academy of Periodontology. Position paper: The role of supra- and subgingival irrigation in the treatment ofperiodontal diseases. J Periodontol. 2005;76:2015–2027.
- Kim AJ, Lo AJ, Pullin DA, Thornton-Johnson DS, Karimbux NY. Scaling and root planing treatment for periodontitis to reduce preterm birth and low birth weight: a systematic review and meta-analysis of randomized controlled trials. J Periodontol. 2012;83:1508–1519.
- American Academy of Periodontology statement on local delivery of sustained or controlled release antimicrobials as adjunctive therapy in the treatment of periodontitis. J Periodontol. 2006;77:1458–1458
- Greenstein G. Local drug delivery in the treatment of periodontal diseases: assessing the clinical significance of the results. J Periodontol. 2006;77:565–578.
- Agarwal E, Bajaj P, Naik SB, Pradeep AR. Locally delivered0.5% azithromycin, as an adjunct to non surgical treatment in chronic periodontitis with type 2 diabetes: a randomized controlledclinical trial. J Periodontol. 2012 Jun 1. [Epub ahead of print]
- Grossi SG, Goodson JM, Gunsolley JC, et al. Mechanical therapy with adjunctive minocycline microspheres reduces red-complex bacteria in smokers. J Periodontol. 2007;78:1741–1750.
- Shaddox, LM, Walker CB. Treating chronic periodontitis:current status, challenges, and future directions. Clin Cosmet Investig Dent. 2010;2:79–91.
- American Academy of Periodontology. Treatment of plaqueinducedgingivitis, chronic periodontitis, and other clinical conditions(position paper). J Periodontol. 2001;72:1790–1800.
- Wilson TG, Glover ME, Malik AK, Schoen JA, Dorsett D.Tooth loss in maintenance patients in a private periodontal practice. J Periodontol. 1987;58:231–235.
- DeVors CH, Duckworth DM, Beck FM, et al. Bone loss following periodontal therapy in subjects without frequent periodontal maintenance. J Periodontol. 1986;57:354–359.
- Becker W, Becker BE, Berg LE. Periodontal treatment without maintenance. A retrospective study in 44 patients. J Periodontol.1984;55:505–509.
- Ramford SP. Maintenance care and supportive periodontal therapy. Quintessence Int. 1993;24:465–471.
- Ramfjord SP, Morrison EC, Burgett FG, et al. Oral hygiene and maintenance of periodontal support. J Periodontol. 1982;53:26–30..
- Cohen RE; Research, Science and Therapy Committee,American Academy of Periodontology. Position paper: periodontal maintenance. J Periodontol. 2003;74:1395–1401.
- Oliver RC, Tervonen T. Diabetes – a risk factor for periodontitisin adults? J Periodontol. 1994;65:530–538.
- Silvestre FJ, Miralles L, Llambes F, Bautista D, Solá-IzquierdoE, Hernández-Mijares A. Type 1 diabets mellitus and periodontal disease: relationship to different clinical variables. Med OralPatol Cir Bucal. 2009;14:E175–E179.
- Ashraf J, Bokhari SAH, Manzoor S, Khan AA. Poor oralhealth and coronary artery disease: a case-control study. J Periodontol.2012;83:1382–1387.
- Teles FR, Teles RP, Martin L, Socransky SS, Haffajee AD.Relationships among interleukin-6, tumor necrosis factor-?,adipokines, vitamin D, and chronic periodontitis. J Periodontol.2012;83:1183–1191.
- Karimbux NY, Saraiya VM, Elangovan S, et al. Interleukin-1gene polymorphisms and chronic periodontitis in adult whites: a systematic review and meta-analysis. J Periodontol.2012;83:1407–1419.
- Michalowicz BS, Novak MJ, Hodges JS, et al. Serum inflammatory mediators in pregnancy: changes after periodontal treatment and association with pregnancy outcomes. J Periodontol.2009;80:1731–1741.
- Offenbacher S, Boggess KA, Murtha AP, et al. Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol. 2006;107:29–36.
- Davies SJ, Gray RJ, Linden GJ, James JA. Occlusal considerations in periodontics. Br Dent J. 2001;191:597–604.
- Lindhe J, Ericsson I. The effect of elimination of jigglingforces on periodontally exposed teeth in the dog. J Periodontol.1982;53:562–567.
- Warnakulasuriya S, Dietrich T, Bornstein M, et al. Oralhealth risks of tobacco use and effects of cessation. Int Dent J.2010;60:7–30.
- Zambon JJ, Grossi SG, Machtei EE, Ho AW, Dunford R,Genco RJ. Cigarette smoking increases the risk for subgingivalinfection with periodontal pathogens. J Periodontol.1996;67:1050–1054.
- Preshaw PM, Heasman L, Stacey F, Steen N, McCracken GI,Heasman PA. The effect of quitting smoking on chronic periodontitis.J Clin Periodontol. 2005;32:869–879.
- McLeod DE, Stoeckel D, Contreras J, Reyes E, Severe postpartumgingival enlargement. J Periodontol.2009;80:1365–1369.
- Consensus report. Surgical pocket therapy. Ann Periodontol.1996;1:618–620.
- Consensus report. Periodontal regeneration around naturalteeth. Ann Periodontol. 1996;1:667–670.
- Consensus report. Mucogingival therapy. Ann Periodontol.1996;1:702–706.
- Epstein SR. Curettage revisited: laser therapy. Pract PeriodonticAesthet Dent. 1992;4:27–32.
- Krause LS, Cobb CM, Rapley JW, Killoy WJ, Spencer P. Laser irradiation of bone. I. An in vitro study concerning the effects of the CO2 laser on oral mucosa and subjacent bone. J Periodontol.1997;68:872–880.
- Moritz A, Schoop U, Goharkay K, et al. Treatment of periodontal pockets with a diode laser. Lasers Surg Med.1998;22:302–311.
- Friesen LR, Cobb CM, Rapley JW, Forgas-Brockman L,Spencer P. Laser irradiation of Bone: II. Healing response following treatment by CO2 and Nd:YAG Lasers. J Periodontol.1999;70:75–83.
- Cobb CM. Lasers in periodontics: a review of literature.J Periodontol. 2006;77:545–564.
- Tomasi C, Schander K, Dahlén G, Wennström JL. Short-termclinical and microbiologic effects of pocket debridement with anEr:YAG laser during periodontal maintenance. J Periodontol.2006;77:111–118.
- Yukna RA, Carr RL, Evans GH. Histologic evaluation of anNd: YAG laser-assisted new attachment procedure in humans.Int J Periodontics Restorative Dent. 2007;27:577–587.
- Armitage GC, Robertson PB. The biology, prevention, diagnosis and treatment of periodontal diseases: scientific advancesin the United States. J Am Dent Assoc. 2009;140(Suppl):36S–43S.
- Shivaprasad B, Pradeep AR. Effect of non-surgical periodontal therapy on interleukin-29 levels in gingival crevicular fluid of chronic periodontitis and aggressive periodontitis patients. DisMarkers. 2013;34:1–7.
- Rios HF. A new model of periodontal susceptibility. Dimensions of Dental Hygiene. 2012;10(10):19–22.
- Benhamou V. Photo disinfection: the future of periodontal therapy. Dent Today. 2009;28:106–109.
- Sharma N, Khuller N. Periodontal vaccine: a new paradigm for prevention of periodontal diseases. J Oral Health Comm Dent.2010;4(Suppl):23–28.
- Dhingra K, Vandana KL. Prophylactic vaccination against periodontal disease: a systematic review of preclinical studies.J Periodontol. 2010;81:1529–1546.
- Burt B; Research, Science and Therapy Committee of theAmerican Academy of Periodontology. Position paper: epidemiology of periodontal diseases. J Periodontol.2005;76:1406–1419.
- Wolff L, Dahlèn G, Aeppli D. Bacteria as risk markers for periodontitis. J Periodontol. 1994;64:498–510.
- Grossi SG, Zambon JJ, Ho AW, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol.1994;65:260–267.
- Grossi SG, Genco RJ, Machtei EE, et al. Assessment of risk for periodontal disease. II. Risk indicators for alveolar bone loss.J Periodontol. 1995;66:23–29.
- American Academy of Periodontology. Non-Surgical PeriodontalTreatment. Available at: www.perio.org/consumer/nonsurgical.Accessed January 16, 2013.
From Dimensions of Dental Hygiene. February 2013; 11(2): 58, 64–67.



