DHDEZVALLE/ISTOCK/GETTY IMAGES PLUS
DHDEZVALLE/ISTOCK/GETTY IMAGES PLUS
Preventing Caries with Peptide-Based Biogenic Products
This emerging technology may provide a minimally invasive solution to caries prevention and management.
This course was published in the March 2022 issue and expires March 2025. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Identify applications for biogenic, peptide-based products in oral healthcare.
- Explain the structure of peptides, as well as their function in dental settings.
- Discuss peptides’ role and mechanism of action in the remineralization process.
Restorative and remineralizing agents continue to be developed to improve patient care and health outcomes. Biogenic peptide-based products are an emerging technology that can serve as a valuable alternative in caries prevention and management (including remineralization), as well as dentinal hypersensitivity treatment. Additionally, their use in dental materials could save time and expense by eliminating the need for traditional treatments—including composite-based restorations—once lesions extend beyond the enamel.1
Biogenic peptide-based formulas are available in clinical materials, as well as over-the-counter (OTC) products designed to support preventive self-care strategies.1 The intent of this article is to inform dental professionals about the structure and properties of peptides, provide an overview of the merits and limitations of peptide-based products in healthcare, and highlight examples of their use in clinical practice.
The inorganic compounds of tooth enamel—the hardest substance in the human body—include calcium, phosphate, and fluoride.2 Enamel contains an acellular organic matrix formulated with amelogenin and highly organized inorganic hydroxyapatite nanocrystals.2 Amelogenin, a noncollagenous protein, is important in regulating the nanocrystalline structure of calcium phosphate, and possesses an amino acid sequence that is needed for crystal growth and enamel formation.2,3 Healthy enamel has a protective covering made of carbonated hydroxyapatite nanorods arranged perpendicularly to the enamel surface and parallel to one another; this creates the aprismatic enamel.4 When a tooth undergoes demineralization, the mineral ions from hydroxyapatite crystals are removed,2 and, due to the inorganic acellular structure, the enamel is unable to regenerate.
Enamel demineralization that has progressed into the dentin is traditionally treated by the excavation of decay and some natural tooth structure, and replacing it with a stable dental material.3 A prominent goal of conservative dentistry is to prevent the loss of tooth structure. Biogenic peptide-based products are organic amino acid proteins used to restore demineralized enamel. These products have been introduced in dentistry as a possible alternative to treat demineralization and prevent further cavitation.3
During tooth development, ameloblasts secrete amelogenin to regulate the growth of hydroxyapatite crystals in the enamel. Peptides containing the amino acid proteins mimicking amelogenin have been shown to positively affect remineralization.3
Peptide Composition
The structure of a peptide is composed of a small number of amino acids linked in short chains termed oligopeptides.5 These chains consist of two main parts: a backbone and side chains.5,6 The backbone is made from a repetitive pattern of amino acids producing a strong ability for hydrogen bonding, with functional groups straying from the backbone to create multiple side chains.5 The hydrogen bonding ability of the peptide backbone uses the calcium and phosphate ions in saliva. The side chains then link together to form a matrix that mimics remineralized enamel.1,5
Mukherjee et al4 identified that certain components of amino acids help shape and stabilize crystals, which can potentially create a denser and more resistant enamel-like foundation. The amino acid peptides were artificially created and arranged in groups of stable nano-spherical assemblies in order to create a dense, threadlike foundation that imitated the structure of enamel and directed the formation of hydroxyapatite crystals. The newly formed crystalline structure aligns in a parallel configuration of aprismatic shapes, closely mimicking the enamel tooth structure. Crystal size influences the properties of the remineralized enamel; smaller apatite crystals exhibit a clinically significant increase in hardness and wear resistance than larger crystals. The unique arrangement of apatite crystals on the enamel provides clinically increased resistance against acids seeking to permeate the tooth structure.4 By imitating the amino acid residues that create the crystalline structure of enamel, a peptide was fabricated to simulate enamel regeneration in cavitated teeth.
In 2019, Zhou et al7 discovered the H5 peptide, which is naturally produced by human salivary glands and was reconstructed by the addition of a phosphoserine group. The modified peptide presented more favorable properties, as the peptide permeated deeper into the demineralized enamel and amplified the antimicrobial properties of saliva. The reconstructed H5 contributed to the destruction of oral bacteria, fungi, and bacterial adhesion within the demineralized lesions.7
Ding et al3 researched another peptide called QP5, which demonstrates the ability to remineralize caries lesions by stabilizing amorphous calcium phosphate and binding the hydroxyapatite crystals to the demineralized tooth surface. When combined with fluoride, the results showed an increase in microhardness and reduced demineralization, as compared to QP5 alone.3
Another self-assembling peptide, P11-4 was investigated by Alkilzy et al8 for treatment of early caries lesions. Through a randomized, controlled, single-blinded study, 70 children with visible caries were separated into a test group treated with P11-4, plus fluoride varnish, and a control group who received only fluoride varnish. The participants were assessed at 3-months and 6-months post-treatment. The peptide P11-4 plus fluoride varnish showed a significantly greater ability to remineralize enamel and prevent further demineralization than fluoride varnish alone. This study supports the use of biogenic peptides in conjunction with fluoride varnish to prevent and treat early lesions.8
![TABLE 1. In-Office Application of Peptide P11-4 Solution]() Use of Peptides in Medicine and Dentistry
Use of Peptides in Medicine and Dentistry
Biogenic peptides are used in different aspects within healthcare (Figure 1), including vaccines, medicine, dentistry, and hemostasis.9 Versions of these biogenic peptides, called virus-like particles (VLP), are used in the human papillomavirus quadrivalent (types 6, 11, 16 and 18) vaccine and hepatitis B vaccine. The VLP’s signal the immune system to create antibodies to fight the virus.9–11 Additionally, biogenic peptides are used in the hemostatic agent RADA 16-I. The ability of RADA 16-I to self-assemble inside of the body provides physical blockage, thereby decreasing bleeding time at the application site.9 RADA 16-I was determined to be clinically effective in reducing bleeding during endoscopic resections.12
Beyond caries management, applications for biogenic peptides have been studied in other uses in oral healthcare, such as treating periodontal diseases. The antibacterial properties of peptides have the potential to target Streptococcus mutans and Porphyromonas gingivalis.13,14 Biogenic peptides have also been studied as a treatment for patients with diabetes and periodontal diseases. Evidence suggests that biogenic peptides could assist in the regulation of the local immune response without creating disruptions within the oral microflora.15
Biogenic peptides can be found in a variety of dental products intended for arresting incipient lesions. A dental biogenic peptide with sodium fluoride contains the peptide P-11, which contributes to remineralization and potential regrowth of the enamel structure.9 Biogenic peptides such as P-11 help promote an environment that further encourages remineralization. The end result is a three-dimensional matrix that mimics the necessary enamel depositional environment.9
Patient Self-Care Application
Peptides can be more broadly considered in clinical application and OTC self-care due to their availability in paste, gel, mouthrinse, and lozenge formulations.16–18 Peptide-based gel products have been shown to reduce dentinal hypersensitivity as efficiently as—or even better than—calcium carbonate toothpaste.18 In this study, the self-care delivery protocol included applying gel to the tooth one to two times a day for 7 days. Based on patient response, the results showed that biogenic peptide-based gel provided more rapid relief; however, further study is needed to support these observations.18

Clinical Application
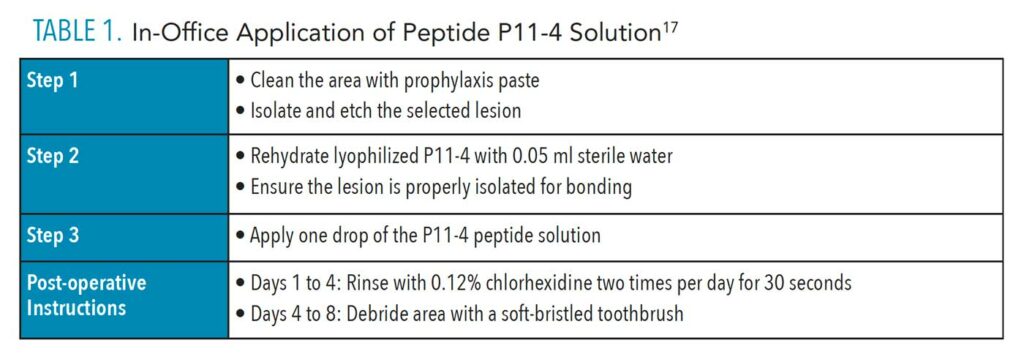
Research completed by Brunton et al17 determined that when P11-4 was exposed to Class V white spot lesions, the peptide draws in minerals that promote hydroxyapatite formation. The authors report that lesions showed statistically significant remineralization after 30 days following a single application.
In this study, application of P11-4 was technique sensitive and required specific patient self-care follow-up (Table 1).17 The clinician first cleaned the affected area with prophylaxis paste, and then isolated and etched the surface area. Next, lyophilized P11-4 was rehydrated with 0.05 ml of sterile water, and one drop of the solution was applied to the lesion. Similar to sealant or composite application, it is necessary for the lesion to be cleaned and isolated to allow adequate bonding of the peptide solution. Post-operative instructions required the patient to avoid brushing that quadrant on days 1 through 4, and only rinse with 0.12% chlorhexidine twice a day for 4 days after application. Patients were instructed to carefully debride the tooth surface using a soft-bristled toothbrush from day 4 to 8 post-operatively, after which they resumed normal oral hygiene procedures.17
Doberdoli et al19 conducted a study in which one group received the P11-4 peptide, along with 900 ppm fluoride varnish, and the second group received the P11-4 peptide, along with an OTC polymeric peptide application. The control group received only fluoride varnish (900 ppm). Results demonstrated that the P11-4 peptide, when used in conjunction with either the self-care polymeric peptide application or fluoride varnish, was more effective in remineralizing incipient caries lesions than what was observed in the control group.
Conclusion
Peptide-based products demonstrate promising results when compared to the use of fluoride alone.8,19 Remineralization of enamel lesions and sensitivity reduction are among the most cited benefits. Favorable qualities include little risk of toxicity, bio-integration of the peptide into enamel, and deep permeability when treating white spot lesions.4,8 On the downside, peptide-based products have some limitations, including limited availability and cost.
Current and emerging research show the potential of biogenic peptides in oral healthcare, and investigators are exploring alternative application methods and evaluating these agents’ success in treating demineralized enamel. Further studies are needed on the long-term effects of managing caries with biogenic peptides. Ultimately, this modality may provide an additional and conservative approach that could help preserve tooth structure.
Acknowledgment: The authors thank Alexandria Nelson, RDH, BSDH, for her assistance in the development of this manuscript.
References
- Holtz J. Peptide-based biogenic dental product may cure cavities. Available at: washington.edu/news/떒/葐/葘/peptide-based-biogenic-dental-product-may-cure-cavities/. Accessed February 14, 2022.
- Abou Neel EA, Aljabo A, Strange A, et al. Demineralization-remineralization dynamics in teeth and bone. Int J Nanomedicine. 2016;11:4743–4763.
- Ding L, Han S, Wang K, et al. Remineralization of enamel caries by an amelogenin-derived peptide and fluoride in vitro. Regen Biomater. 2020;7:283–292.
- Mukherjee K, Ruan Q, Nutt S, Tao J, De Yoreo JJ, Moradian-Oldak J. Peptide-based bioinspired approach to regrowing multilayered aprismatic enamel. ACS Omega. 2018;3:2546–2557.
- Berg JM, Tymoczko JL, Stryer L. Chapter 3: Protein Structure and Function. In: Berg JM, Clarke ND, Stryer L, Tymoczko JL. Biochemistry. 5th ed. New York: WH Freeman; 2002.
- Carugo O. Amino acid composition and protein dimension. Protein Sci. 2008;17:2187–2191.
- Zhou L, Wong HM, Zhang YY, Li Li Q. Constructing an antibiofouling and mineralizing bioactive tooth surface to protect against decay and promote self-healing. ACS Appl Mater Interfaces. 2020;12:3021–3031.
- Alkilzy M, Tarabaih A, Santamaria RM, Splieth CH. Self-assembling peptide P11-4 and fluoride for regenerating enamel. J Dent Res. 2018;97:148–154.
- Hainline KM, Fries CN, Collier JH. Progress toward the clinical translation of bioinspired peptide and protein assemblies. Adv Healthc Mater. 2018;7:1700930.
- United States Food and Drug Administration. Gardasil. Available at: fda.gov/vaccines-blood-biologics/vaccines/gardasil. Accessed February 14, 2022.
- United States Food and Drug Administration. Cervarix. Available at: fda.gov/vaccines-blood-biologics/vaccines/cervarix. Accessed February 14, 2022.
- Subramaniam S, Kandiah K, Thayalasekaran S, Longcroft-Wheaton G, Bhandari P. Haemostasis and prevention of bleeding related to ER: The role of a novel self-assembling peptide. United European Gastroenterol J. 2019;7:155–162.
- National Maternal and Child Oral Health Resource Center. 3.2 Stages in Caries Lesion Severity and Activity. Available at: mchoralhealth.org/Dental-Sealant/䁱-tooth-selection/䁱-2.php. Accessed February 14, 2022.
- Alaei SR, Park JH, Walker SG, Thanssi DG. Peptide-based inhibitors of fimbrial biogenesis in Porphyromonas gingivalis. Infect Immun. 2019;87:e00750-18.
- Arobidin ogli UB. Use of biogenic peptides in complex treatment of inflammatory diseases of paradont in patients with diabetes mellitus II type. Available at: repo.journalnx.com/index.php/nx/article/view/릩. Accessed February 14, 2022.
- Pepperney A, Chikindas ML. Antibacterial peptides: Opportunities for the prevention and treatment of dental caries. Probiotics Antimicrob Proteins. 2011;3:68–96.
- Brunton PA, Davies RP, Burke JL, et al. Treatment of early caries lesions using biomimetic self-assembling peptides—a clinical safety trial. Br Dent J. 2013;215:E6.
- Schlee M, Rathe F, Bommer C, Bröseler F, Kind L. Self-assembling peptide matrix for treatment of dentin hypersensitivity: A randomized controlled clinical trial. J Periodontol. 2018;89:653–660.
- Doberdoli D, Bommer C, Begzati A, Haliti F, Heinzel-Gutenbrunner M, Juric H. Randomized clinical trial investigating self-assembling peptide P11-4 for treatment of early occlusal caries. Sci Rep. 2020;10:4195.
From Dimensions of Dental Hygiene. March 2022;20(3):38-41.