
Pain Control for Pediatric Patients
Clinicians should be well versed in the appropriate methodology, equipment, and drug selection when administering local anesthesia to children.
The ability to provide safe and effective local anesthesia is one of the most important skills required of dental practitioners.1 In order to achieve this skill set, oral health professionals must be aware of differences in patient populations that may alter the methodology, equipment, and selection of agents necessary for effective pain control. While there are more similarities than differences with adult vs pediatric local anesthesia administration, this article will focus on some specific concerns that can occur with pediatric local anesthesia delivery.
COMMUNICATION AND BEHAVIOR
Dental visits can have serious emotional consequences for children. Lifelong feelings of anxiety and fear about dental care are often linked to negative experiences that occurred during adolescence.2 Local anesthetic injections can be painful, and trepidation about injections can delay necessary treatment.3,4
The clinician’s ability to effectively and accurately communicate with patients is vital to creating a positive experience and favorable outcomes. The avoidance of trigger words, such as shot, bee sting, needle, pinch, etc, is advised during pediatric interaction. Additionally, there are three verbal approaches to patient/parent/caregiver fear that have been proposed, including: the permissive approach (provide relevant information regarding treatment to relieve uncertainty); empathetic approach (share another person’s feelings); and personal approach (create the feeling that a personal relationship exists). Each approach should be used when generally communicating with children and their parents/caregivers. The empathetic approach (eg, “This may be a little uncomfortable and I know you are scared, but we are going to do all we can to help you,” or “It is alright to be afraid; I know I get a little scared at the dentist, too.”) is the most effective in reducing anxiety during the administration of painful stimuli.5–7
In addition to fostering good provider-patient communication, oral health professionals should employ behavior management techniques to encourage patient cooperation. One of the most common techniques used in dental local anesthesia administration is vocal distraction—or the method of focusing the patient on the provider’s voice rather than on the procedure.8 When a child has the opportunity to focus on other things, injection completion is more likely to occur.8
Controlled stimulation is another technique. It involves the manipulation of tissues and/or affiliated nerve endings in a manner that elicits a distraction from painful stimuli. Controlled stimulation devices that provide auditory, vibratory, or visual distraction are available that may help children cope with receiving injections.
DRUG TOXICITY/OVERDOSE
One of the most important considerations when treating children is the potential for drug overdose. In dentistry, dose-dependent toxicity is most frequently reported with pediatric care.8–10 Clinicians need to identify the risks associated with anesthetic administration and understand the maximum recommended doses of local anesthetic agents. Challenges exist, however, to ensuring appropriate dosing. One such obstacle is obesity, which is a complicating factor during dental care.11–15 This is due to an increased presence of adipose tissue in the oral cavity, which makes it difficult to properly identify a patient’s intraoral anatomy.14 Obesity may also alter drug distribution and elimination, which can affect appropriate dosing and result in complications.11,12 The use of standard dose-per-pound criteria may not result in an accurate administration of the agent.
Unfortunately, there are no specific guidelines for local anesthetic dosing in obese children.8 Medical-based research proposes the use of ideal body weight (IBW) when calculating doses for obese patients.16,17 IBW is a statistical formula that uses combinations of height, weight, gender, and/or frame size to determine optimal body weight. Several IBW graphs are available online, and many smartphones now include downloadable IBW applications.
An analysis of the different anesthetic agents used for pediatric local anesthesia reveals that 3% mepivacaine without epinephrine is associated with a larger number of toxicity reports compared to other anesthetic agents.8,9,18–20 These increased toxicity reports may relate to the lack of a vasoconstrictor (epinephrine or levonordefrin), which can create an environment of increased systemic absorption. Pharmacokinetic evaluation has revealed that anesthetic blood levels of 3% mepivacaine without epinephrine peak at a more rapid rate when compared with an equal amount of 2% lidocaine with 1:100,000 epinephrine.21–23 Therefore, the benefits of using 3% mepivacaine in a pediatric population should be weighed against known risks.
POST-ANESTHETIC TRAUMA
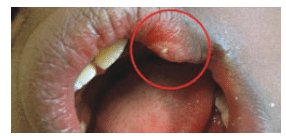
The use of 3% mepivacaine alone in pediatric dentistry is popular because its duration is thought to be briefer than other agents. A shorter duration of anesthesia can reduce the occurrence of severe post-anesthetic trauma, such as self-inflicted soft-tissue injuries. While the durations of pulpal anesthesia with mepivacaine without epinephrine are shorter than those of 2% lidocaine with 1:100,000 epinephrine, the length of soft-tissue anesthesia for each agent is nearly identical.23,24 Soft-tissue anesthesia is responsible for nearly all biting and chewing injuries.15
A considerable concern with pediatric oral anesthesia is the occurrence of self-inflicted soft-tissue injuries, such as lip bites (Figure 1). This injury is one of the most common local anesthesia complications experienced by adolescents. Self-inflicted soft-tissue trauma following local anesthetic administration occurs from 3% to 16% of the time.15,25 This complication is usually reported as a mild or moderate incident; however, severe events have been described, including amputation of the lower lip.15,26 If notified of a self-inflicted soft-tissue injury within 16 hours of an appointment, clinicians should recommend placing an ice pack over the injured tissue to reduce edema.8 If notification occurs the day following an appointment, a warm pack can be recommended to stimulate circulation and promote healing.8 If the patient returns to the dental office for evaluation, anecdotal reports have suggested that the application of 0.12% chlorhexidine solution to the outer area of the tissue trauma with cotton-tipped applicators improves healing by reducing bacterial levels.8
REVERSAL OF LOCAL ANESTHESIA
The use of phentolamine mesylate (PM) to reverse soft-tissue anesthesia in the oral cavity was granted United States Food and Drug Administration approval for dental application in 2008. PM is an alpha-adrenergic blocker that completely obstructs pre- and post-synaptic adrenergic receptors. In dentistry, PM is used to decrease the duration of soft-tissue anesthesia after local anesthetic administration by increasing the elimination of anesthetic from the localized area via a reduction in localized vascular resistance.27 In other words, PM works by affecting the alpha receptor on a blood vessel. When active, the alpha receptor will cause the blood vessel to constrict, which reduces the ability of the material in the tissues to leave the area via the blood stream. PM inhibits the ability of the alpha receptor to signal the vessel to constrict or continue with constriction. Thus, the materials in the tissues have a greater ability to leave the area into the bloodstream. The research concludes that the use of PM reduced soft-tissue anesthesia and accelerated patients’ return to normal lip sensation within approximately 1.4 hours following mandibular block and maxillary infiltration injections, and improved the return to normal tongue sensation by approximately 1.1 hours following mandibular block injections.28,29
A recent study evaluating complication rates with local anesthetic administration and reversal revealed an improvement in safety outcomes when PM was administered to pediatric dental patients.15 The patients who did not receive PM experienced the majority of soft-tissue injuries and demonstrated a higher incidence of complications overall. Additionally, the authors found a higher incidence of self-inflicted soft-tissue trauma in patients with attention deficit hyperactivity disorder and those considered overweight/obese who did not receive PM.15
PM is administered in 0.4 mg cartridges at a 1:1 ratio of the agent to local anesthetic cartridge at the local anesthetic injection site. The agent is available for use in all patients who meet the age and weight requirements. The maximum dose is two cartridges, and the agent is not recommended in patients under the age of 6 or who weigh less than 33 lbs. A clinical trial in 2-year-old to 5-year-old subjects is currently being conducted to establish safety and efficacy.
CONCLUSION
Considerations for pediatric local anesthetic administration include psychological management, toxicity, and soft-tissue injuries. Additionally, there are technique variations that relate to skull size and anatomical differences that result in decreased depth of injection and easier distribution of anesthetic due to less dense bone. Ultimately, small nuances between pediatric and adult local anesthesia administration exist. It is imperative to assess each pediatric patient’s weight for local anesthetic administration by using an appropriate in-office scale. In addition, being able to make the distinctions with alterations in methodology, devices, and agents will result in a higher level of patient satisfaction and improved outcomes.
REFERENCES
- Moore PA, Hersh EV, Boynes SG. Preface update of dental local anesthesia. Dent Clin N Am. 2010;54:xiii–xiv.
- Milsom KM, Tickle M, Humphris GM, Blinkhorn AS. The relationship between anxiety and dental treatment experience in 5-year-old children. Br Dent J. 2003;194:503–506.
- Matthews DC, Rocchi A, Gafni A. Factors affecting patients’ and potential patients’ choices among anesthetics for periodontal recall visits. J Dent. 2001;29:173–179.
- Sokolowski CJ, Giovanniti JA, Boynes SG. Needle phobia: etiology, adverse consequences, and patient management.Dent Clin N Am. 2010;54:731–744.
- Sarnat H, Arad P, Hanager D, Shohami E. Communication strategies used during pediatric dental treatment: a pilot study. Pediatr Dent. 2001;23:337–342.
- Hooper EM, Cornstock LM, Goodwin JM, Goodwin JS. Patient characteristics that influence physician behavior. Med Care. 1982;20:630–638.
- Steward DJ, Lerman J. Manual of Pediatric Anesthesia. 5th ed. Philadelphia: Churchill Livingstone; 2001.
- Bassett KB, DiMarco AC, Naughton DK. Local Anesthesia for Dental Professionals. 2nd ed. Upper Saddle River, New Jersey: Prentice Hall; 2014.
- Goodson JM, Moore PA. Life-threatening reactions following pedodontic sedation: an assessment of narcotic, local anesthetic, and antiemetic drug interaction. J Am Dent Assoc. 1983;107:239–245.
- Reynolds F. Adverse effects of local anesthetics. Br J Anaesth. 1987;59:78–95.
- Cheymol G. Effects of obesity on pharmacokinetics implications for drug therapy. Clin Pharmacokinet. 2000;39:215–231.
- Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public health crisis, common sense cure. Lancet. 2002;360:473–482.
- Pischon N, Heng N, Bernimoulin JP, Kleber BM, Willich SN, Pischon T. Obesity, inflammation, and periodontal disease. J Dent Res. 2007;86:400–409.
- Reilly D, Boyle CA, Craig DC. Obesity and dentistry: a growing problem. Br Dent J. 2009;207:171–175.
- Boynes SG, Riley AE, Milbee S, Bastin MR, Price ME, Ladson A. Evaluating complications of local anesthesia administration and reversal with phentolamine mesylate in a portable pediatric dental clinic. Gen Dent. 2013;61:70–76.
- Bouillon T, Shafer SL. Does size matter? Anesthesiology. 1998;89:557–560.
- Leykin Y, Pellis T, Lucca M, Lomangino G, Marzano B, Gullo A. The pharmacodynamic effects of rocuronium when dosed according to real body weight or ideal body weight in morbidly obese patients. Anesth Analg. 2004;99:1086–1089.
- Hersh EV, Helpin ML, Evans OB. Local anesthetic mortality: report of case. ASDC J Dent Child. 1991;58:489–491.
- Moore PA, Goodson JM. Risk appraisal of narcotic sedation for children. Anesth Prog. 1985;32:129–139.
- Virts Be. Local anesthesia toxicity review. Pediatr Dent. 1999;21:375.
- Goebel W, Allen G, Randall F. Circulating serum levels of mepivacaine after dental injection. Anesth Prog. 1978;25:52–56.
- Goebel WM, Allen G, Randall F. The effect of commercial vasoconstrictor preparations on the circulating venous serum level of mepivacaine and lidocaine. J Oral Med. 1980;35:91–96.
- Moore PA, Hersh EV. Local anesthetics: pharmacology and toxicity. Dent Clin N Am. 2010; 54:587–599.
- Hersh EV, Hermann DG, Lamp CL, Johnson PD, MacAfee KA. Assessing the duration of mandibular soft tissue anesthesia. J Am Dent Assoc. 1995;126:1531–1536.
- Chi D, Kanellis M, Himadi E, Asselin ME. Lip biting in a pediatric dental patient after dental local anesthesia: a case report. J Pediatr Nurs. 2008;23:490–493.
- Akram A, Kerr RMF, Mclennan AS. Amputation of lower left lip following dental local anesthetic. Oral Surgery. 2008;1(2):111–113.
- Moore PA, Hersh EV, Papas AS, et al. Pharmacokinetics of lidocaine with epinephrine following local anesthesia reversal with phentolamine mesylate. Anesth Prog. 2008;55:40–48.
- Hersh EV, Moore PA, Papas AS, et al. Reversal of soft-tissue local anesthesia with phentolamine mesylate in adolescents and adults. J Am Dent Assoc. 2008;139:1080–1093.
- Tavares M, Goodson JM, Studen-Pavolich D, et al. Reversal of soft-tissue anesthesia with phentolamine mesylate in pediatric patients. J Am Dent Assoc. 2008;139:1095–1104.
From Dimensions of Dental Hygiene. October 2014;12(10):32–33,36.

