 TAKOBURITO/ ISTOCK/ THINKSTOCKK
TAKOBURITO/ ISTOCK/ THINKSTOCKK
Oral Health Risks in Athletes
While athletes are in prime physical condition, they may be at increased risk for bruxism, dental erosion, and xerostomia.
This course was published in the August 2016 issue and expires August 31, 2019. The authors have no commercial conflicts of interest to disclose. This 2 credit hour self-study activity is electronically mediated.
EDUCATIONAL OBJECTIVES
After reading this course, the participant should be able to:
- Discuss the relationship between bruxism and weightlifting.
- Explain why competitive swimmers are at increased risk of dental erosion.
- Identify the reasons athletes may be more susceptible to xerostomia than nonathletes.
- List strategies for reducing these oral health risks among athletes.
The training required of both professional and recreational athletes is physically demanding. While sore muscles and overuse injuries are common among athletes, oral health can also be adversely affected. Research conducted by Needleman et al1 during the 2012 Olympic Games in London revealed that competing athletes’ oral health was poor. The causes behind this varied among sport categories and training regimens. The most prevalent problems, however, were consistently preventable (Table 1). Needleman et al1 also determined that poor oral health negatively impacted athletes’ training and performance.
Bruxism and dental erosion can be caused by athletes’ training regimens. Additionally, many sports, such as endurance running and high-intensity martial arts, encourage mouth breathing due to the body’s increased need for oxygen during activity. Mouth breathing may lead to xerostomia.
BRUXISM AND WEIGHTLIFTING
Bruxism is defined as the clenching, bracing, or grinding of the teeth.2,3 The masseter muscle forcefully closes the maxilla with the mandible (Figure 1). Studies have shown that the masseter demonstrates paired activity with muscles in the legs, arms, and back.4–6 As a result, activation of muscles in the legs, arms, and back can be accompanied by involuntary activation of the masseter muscle. Consequently, weightlifting, which demands intense strength of these muscles, is often accompanied by clenching.6
Due to the nature of their sport, powerlifters are at high risk of long-term damage to their dentitions. Powerlifting is a competitive sport in which individuals perform three fundamental lifts: squat, bench press, and deadlift. The goal is to complete each lift with the highest weight, determined by an individual’s weight class.7 Two lifts engage the vastus lateralis muscle, and all three require additional effort as the weight load increases.
Huang et al8 demonstrated, through measurement of electromyography activity at various workloads, that increased power output yielded increased engagement of the masseter during leg muscle activity. The researchers found that facial muscle activity increased significantly during high-intensity exercise. Further, activity of the masseter displayed a strong positive correlation with heart rate, perceived exertion, and activity of the vastus lateralis muscle. The study suggested that the masseter muscle is likely to contract during exercises that require engagement of the vastus lateralis, such as squats and deadlifts, and the force of contraction increases with effort. As such, the activity of the masseter results in bruxing.8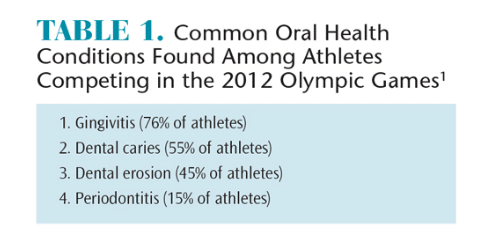
Studies have shown that voluntary clenching enhances body stability, which is required in weightlifting.4,5 Hellman et al5 demonstrated this concept through the measurement of range of motion and angular velocities of the ankle, knee, and hip joints in a narrow stance during various occlusal loads. These measurements showed a smaller range of motion during occlusal load, suggesting that a greater biting force stabilizes an individual’s stance.4 As such, bruxing during squats and deadlifts—which require body stability—may help the athlete achieve success at heavier weight loads.
Chronic bruxism contributes to a number of dental conditions, including temporomandibular disorders (TMD), attrition, tooth mobility, and changes to the alveolar bone.2 Chronic, intense bruxing generates great occlusal force on the periodontal ligament that may result in alveolar bone loss, leading to tooth mobility. Pergamalian et al9 reported that 26% to 66% of patients with TMD were self-reported bruxers. Intense contraction of the mastication muscles—which is required during bruxing—generates muscle irritation that may cause pain in the preauricular area, reduced range of motion within the temporomandibular joint and crepitus during mandibular function. Further, these symptoms can exacerbate headaches, neck aches, earaches, and other facial pain.9 Although current literature suggests an association between bruxing and attrition, the multifactorial etiology of hard tissue destruction makes determining the exact impact of bruxing challenging. Additionally, enamel wear occurs naturally with age, further complicating the causative relationship between bruxing and attrition.9
SCIENCE PICTURE CO/SCIENCE PHOTO LIBRARY
Severe chronic bruxing can also damage restorations. Failure of dental restorations is most frequently attributed to loss of retention or a fracture in the restorative material or the tooth itself. In addition, achieving the necessary mechanical retention for a successful restoration on a natural crown shortened by attrition is difficult. Furthermore, teeth restored after bruxing are at an increased risk for caries and endodontic involvement. While the sequence of events is uncertain, restorations on shortened crowns yield microleakage and may result in recurrent caries and marginal degradations.2
Resin-based restorations are at increased risk of fracture due to the strong occlusal force applied by those who brux. Accordingly, metal-ceramic restorations or full-metal restorations should be chosen for patients who are chronic bruxers. Similarly, multi-unit restorations should be constructed as single crowns whenever possible to decrease torque forces during bruxing.2 Research regarding the effect of bruxism on dental implant prognosis remains controversial. Several studies suggest, however, that bruxing may yield a higher incidence of implant complications.2,6
Devices are available to avoid impairment of dentition in weightlifters at risk for bruxing. For example, occlusal splints, such as mouth guards, may be worn to prevent attrition and decrease occlusal forces. Additionally, behavior modifications may decrease the frequency of bruxing, as well.2 For instance, lifters may exhale as they complete their movement to prevent excessive clenching. However, this precautionary action may, in turn, decrease the power of their lift.
While occasional bruxing may not cause long-term damage to the oral cavity, chronic bruxing increases the risk of a number of dental conditions. Attrition is a slow process that occurs over time. Therefore, with proper intervention— such as the use of an occlusal splint and appropriate behavior modification—long-term damage may be prevented.2
DENTAL EROSION IN COMPETITIVE SWIMMERS
Dental erosion is the loss of tooth structure due to acidic chemical reactions (Figure 2).10 The severity of dental erosion is multifactorial and depends on the type and time of exposure to an erosive agent, mineralization of dental tissue, and salivary composition.11 This condition is common in competitive swimmers due to inadequate buffering of chlorine in pool water.9 Chlorine compounds are used to disinfect swimming pool water. Gas chlorination and trichloroisocyanuric acid (TCCA) tablets are the most common disinfection techniques. Gas chlorination results in the formation of hydrochloric acid in the water.12 Inadequate buffering of hydrochloric acid can decrease the pH level significantly, increasing the risk of dental erosion on contact with the teeth.13 TCCA tablets produce hypochlorous acid (HOCl) and cyanuric acid. HOCl presents no effect on humans; however, cyanuric acid stabilizes chlorine in the presence of sunlight. This allows chlorine to accumulate in the water, making pool water more acidic and increasing swimmers’ risk of dental erosion.12
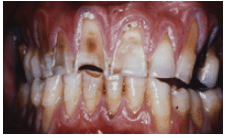
BIOPHOTO ASSOCIATES/SCIENCE PHOTO LIBRARY
Current literature demonstrates gas chlorination enhances dental erosion. Gas chlorination forms hydrochloric acid, which decreases the pH level of pool water.12 In the absence of proper buffering, the pH can drop as low as 3.0. Demineralization of enamel can begin at a pH level of 5.5. Therefore, contact of enamel with inadequately buffered pool water treated through gas chlorination will yield dental erosion. A Polish study analyzed the prevalence of dental erosion in adolescent competitive swimmers exposed to gas-chlorinated swimming pool water.10 Results revealed that dental erosion was observed in more than 26% of the competitive swimmers and 10% of the recreational swimmers studied. Furthermore, the lesions in competitive swimmers were present on the labial and palatal surfaces of anterior teeth.10 These results are consistent with the current literature, which states that the maxillary and mandibular anterior teeth of swimmers are most commonly affected by dental erosion.14
Unlike the direct action of gas chlorination byproducts on enamel, TCCA treatment exerts an indirect effect. Cyanuric acid, generated by the TCCA treatment, causes pool water to accumulate a greater concentration of chlorine. The chlorine then lowers the pH of pool water and causes dental erosion on contact with the teeth.12 Chuenarrom et al12 investigated the effects of excessive use of TCCA in swimming pool water on dental erosion. Researchers determined that when enough TCCA was used to decrease the pool water’s pH to levels below 5.0, dental erosion occurred in swimmers.12 Additionally, a 2011 clinical report described the presentation of a 52-year-old man with extremely sensitive teeth.15 The etiology of this patient’s sensitivity was rapid dental erosion. After assessment of the dental history, it was noted that he had a 90-minute daily swimming routine. TCCA tablets were used to chlorinate the pool he used. Because the pool water was inadequately buffered, a low pH environment resulted, leading to the subsequent diagnosis of dental erosion.15
Dental erosion can cause diastema due to a loss of tooth structure, prominent margins of the lesion that resemble anterior veneer preparations, wear of the incisal edge, and dentinal hypersensitivity. While dental erosion is irreversible, fluoride has been shown to effectively manage side effects and prevent further damage.11,16 Studies have shown that the use of topical fluoride treatment results in the formation of a protective barrier over tooth enamel. This not only alleviates sensitivity, but it also inhibits further loss of tooth structure.16 Therefore, fluoride may be used as a preventive treatment in competitive swimmers. As previously described, the original tooth structure cannot be regenerated. However, restorative treatments, such as direct composite resin restorations, porcelain laminated veneers, and complete coverage crowns, are available to improve the function and esthetics of severely eroded teeth.14
XEROSTOMIA AND MOUTH BREATHING
Many athletes experience varying levels of xerostomia and changes in salivary components during exercise.17 The heart muscles contract more frequently and forcefully during high-intensity exercise, generating a need for more oxygen and energy.18 This need for oxygen causes athletes to breathe through their mouths, which is a primary cause of xerostomia.19 Xerostomia increases an athlete’s susceptibility to plaque accumulation, and can result in gingivitis and severe tooth decay.20 Additionally, changes in salivary composition during intense exercise have been associated with biofilm accumulation and increased caries risk.21
Current literature reports that salivary flow rates decrease during intense exercise in response to hyperventilation and mouth breathing.22,23 Ljungberg et al24 measured salivary production in runners before and after completing a marathon. Results showed that the salivary flow rate was significantly lower after the race than before.24 Likewise, a more recent study by Mulic et al11 investigated salivary flow during exercise and revealed a flow decrease in more than half of participants.
In addition to xerostomia, athletes who breathe through their mouths may exhibit alterations in their salivary components.21,25 Saliva is primarily water but also contains immunoglobulins, proteins, enzymes, mucins, urea, and ammonia.26 The concentration of each component can vary with changes in salivary flow rate during exercise. Concentration variance alters the antibacterial mechanisms of saliva, increasing the athlete’s susceptibility to biofilm accumulation.21 As bacteria accumulate and produce acidic toxins, dental caries may occur.
Kiania et al21 measured the effect of varying intensities of aerobic activity on salivary protein concentration. Results revealed that the average protein concentration decreased immediately after exercise, and further decreased 1 hour after the cessation of exercise. Salivary proteins contain antimicrobial properties, which are vital to maintaining oral tissues.21 Therefore, changes in salivary protein concentration during prolonged intense exercise may alter the antimicrobial properties of the saliva, leading to an increase in caries risk.
Sialic acid is a component of salivary glycoproteins, which enhances bacterial aggregation, as well as the formation of the acquired pellicle and dental biofilm. Weiler et al25 demonstrated that mouth breathing significantly increased the concentration of free sialic acid in unstimulated saliva. Increased concentration of this acid encourages the proliferation of oral bacteria in mouth-breathing individuals. Additionally, this study suggested that an increase in acid concentration is caused by the evaporation of water content from saliva. As such, it can be hypothesized that xerostomia due to mouth breathing may demonstrate the same effect.
Although studies directly assessing athletes and their breathing habits remain limited, current research suggests that mouth-breathing individuals demonstrate higher levels of sialic acid, which increases bacterial aggregation, than nonmouth breathers. Therefore, athletes may experience high levels of sialic acid due to mouth breathing during their workouts. More research is needed to better understand the relationship between xerostomia and high-intensity exercise.
Xerostomia may be treated with a variety of products, including pharmaceutical agents and salivary substitutes. However, salivary stimulation is the preferred intervention for xerostomia.27,28 Moreover, the xerostomia experienced by athletes during exercise is limited to the duration of their workouts. Therefore, salivary stimulation during workouts is likely the most effective form of intervention.
Gum chewing stimulates saliva in the oral cavity. Nogourani et al28 investigated the effect of various flavors of sugar-free chewing gum on salivary flow rate and salivary pH. Results demonstrated that spearmint, cinnamon, and fruit-flavored chewing gums all produced significant increases in flow rate and the pH of saliva.28 Another study showed stimulation through chewing gum during exercise boosts ?-amylase and lysozyme secretion.29 This enhances the antimicrobial action of saliva and inhibits bacterial accumulation. Sugar-free lozenges effectively stimulate saliva production, as well.30 None of the resources examined mentioned hazards associated with choking from swallowing the chewing gum while running. This is an area for further investigation.
CONCLUSION
Despite the risk factors presented by an athletic lifestyle, preventive methods are available to maintain oral health. Nonintrusive behavior modifications—such as implementing proper breathing, wearing an occlusal guard, using fluoride, and chewing sugar-free gum—may help minimize oral health risks. Future studies are necessary to obtain stronger evidence of the oral health hazards presented by an athletic lifestyle. By building a body of evidence supporting behavior modifications, athletes may be more likely to comply with recommendations and strive to achieve optimal oral health.
References
- Needleman I, Ashley P, Petrie A, et al. Oral healthand impact on performance of athletes participating in the London 2012 Olympic Games: a cross-sectional study. Br J Sports Med. 2013:47:1054–1058.
- Johansson A, Omar R, Carlsson G. Bruxism andprosthetic treatment: A critical review. J Prosthodont Res. 2011:55:127–136.
- Murali RV, Rangarajan P, Mounissamy A. Bruxism:conceptual discussion and review. J Pharm Bioallied Sci. 2015:7:265–270.
- Hellmann D, Giannakopoulos NN, Blaser R, Eberhard L. The effect of various jaw motor tasks on body sway. J Oral Rehab. 2011:38:729–736.
- Ringhof S, Leibold T, Hellmann D, Stein T. Posturalstability and the influence of concurrent muscle activation—beneficial effects of jaw and fist clenching. Gait Posture. 2015:10;42:598–600.
- Varalakshmi Reddy S, Praveen Kumar M,Sravanthi D, Habeeb Bin Mohsin A, Anuhya V. Bruxism: a literature review. J Int Oral Health. 2014;6:105–109.
- Kozub FM, Brusseau TA. Powerlifting. Journal of Physical Education, Recreation & Dance .2012:83(3):34–41.
- Huang D, Chou S, Chen Y, Chiou W. Frowningand jaw clenching muscle activity reflects the perception of effort during incremental workload cycling. J Sports Sci Med. 2014:13:921–928.
- Pergamalian A, Rudy T, Zaki H, Greco C. Theassociation between wear facets, bruxism, and severity of facial pain in patients with temporomandibular disorders. J Prosthet Dent. 2003:90:194–200.
- Buczkowska-Radli?ska J, Lagocka R, Kaczmarek W, Gorski M, Nowicka A. Prevalence of dental erosion in adolescent competitive swimmers exposed to gas-chlorinated swimming pool water. Clin Oral Invest. 2012:17:579–583.
- Mulic A, Tveit AB, Songe D, Sivertsen H, Skaare AB. Dental erosive wear and salivary flow rate in physically active young adults. BMC Oral Health. 2012:12:8.
- Chuenarrom C, Daosodsai P, Charoenphol P. Effects of excessive trichloroisocyanuric acid in swimming pool water on tooth erosion. Songklanakarin J Sci Technol. 2011;36:445–450.
- Zebrauskas A, Birskute R, Maciulskiene V. Prevalence of dental erosion among the young regular swimmers in Kaunas, Lithuania. J Oral Maxillofac Res. 2014:5:e6.
- Peampring C. Restorative management using hybrid ceramic of a patient with severe tooth erosion from swimming: a clinical report. J Adv Prosthodont. 2014:6:423–426.
- Jahangiri L, Pigliacelli S, Ross Kerr A. Severe and rapid erosion of dental enamel from swimming: A clinical report. J Prosthet Dent. 2011:106:219–223.
- Lussi A. Introduction. Stabilized stannous fluoride and dental erosion. Int Dent J. 2014:64:2–3.
- Frese C, Frese F, Kuhlmann S, et al. Effect ofendurance training on dental erosion, caries, and saliva. Scand J Med Sci Sports. 2015:25:319–326.
- LeMond G, Hom M. Your body at work, play, and rest. The Science of Fitness: Power, Performance, and Endurance. Atlanta: Elsevier Science; 2015:71–86.
- Farsi N. Signs of oral dryness in relation tosalivary flow rate pH buffering capacity and dry mouth complaints. BMC Oral Health. 2007:7:15–20.
- Ekström J, Khosravani N, Castagnola M, MessanaI. Saliva and the control of its secretion. In: Dysphagia. Diagnosis and Treatment. New York: Springer; 2012:19–47.
- Kiani M, Geramian Z, Saboori M, Shahed A.Effect of different intensity aerobic activity on total protein concentration in saliva. International Journal of Applied Exercise Physiology. 2015:4(1):18–24.
- Chicharro JL, Lucía A, Pérez M, Vaquero AF, Ureña R. Saliva composition and exercise. Sports Med. 1998:26:17–27.
- Dawes C. How much saliva is enough for avoidance of xerostomia? Caries Res. 2004:38:236–240.
- Ljungberg G, Ericson T, Ekblom B, Birkhed D.Saliva and marathon running. Scand J Med Sci Sports. 1997:7:214–219.
- Weiler RME, Fisberg M, Barroso AS, Nicolau J,Simi R, Siqueira WL. A study of the influence of mouth-breathing in some parameters of unstimulated and stimulated whole saliva of adolescents. Int J Pediatr Otorhinolaryngol. 2006:70:799–805.
- Humphrey SP, Williamson RT. A review of saliva:normal composition flow and function. J Prosthet Dent. 2001:85:162–169.
- Rieger JM. Recent advances in the prevention and treatment of xerostomia: a review of the literature. Canadian Journal of Dental Hygiene. 2012:46(3):159–165.
- Nogourani MK, Janghorbani M, Isfahan RK,Beheshti MH. Effects of chewing different flavored gums on salivary flow rate and pH. Int J Dent. 2012;2012:569327.
- Allgrove JE, Oliveira M, Gleeson M. Stimulating whole saliva affects the response of antimicrobial proteins to exercise. Scand J Med Sci Sports. 2014:24:649–655.
- Epstein JB, Beier Jensen S. Management of hyposalivation and xerostomia: criteria for treatment strategies. Compend Contin Educ Dent. 2015:36:600–603.
From Dimensions of Dental Hygiene. August 2016;14(08):74–77.



